Effect of pH value and Fe(Ⅲ) on the oxidative dissolution of stibnite
-
摘要: 矿山开采活动影响下辉锑矿的氧化溶解是影响岩-土-水环境介质中锑的迁移转化及其环境效应的重要过程。目前对于辉锑矿溶解的研究主要关注动力学特征,对于它氧化溶解的途径、环境因素的影响、锑的释放规律等重要问题的认识还不明确。为探究碳酸盐岩矿区地下水中锑释放过程,选取重要环境因素pH值和Fe(Ⅲ),采用单因素控制条件下的批实验方法,精细刻画避光条件下辉锑矿(Sb2S3)氧化溶解速率及Sb和S氧化产物的组成特征。研究结果表明,Sb2S3的氧化溶解是一个产酸的过程,Sb和S的释放速率、途径和产物特征受pH值和Fe(Ⅲ)的显著影响。Sb2S3的氧化溶解速率由快变慢后趋于平衡,初始反应速率的量级为10-8 mol/(m2·s),平衡反应速率的量级为10-10 mol/(m2·s)。Sb的释放氧化速率随pH值的增加而增加,强碱条件下最有利于Sb的释放和氧化。强酸条件下,H2S、SO2气体逸出和S(0)的沉淀促进了Sb2S3的溶解,Sb(Ⅲ)和S(0)为主要产物。中性条件下,溶解形成的HS-经逐步氧化生成SO42-和少量S2O32-,Sb(Ⅲ)和Sb(Ⅴ)含量相近。强碱条件下,SbS33-和Sx-的生成显著提升了Sb2S3的氧化溶解速率,Sb(Ⅴ)和S2O32-是主要产物。Fe(Ⅲ)单独氧化作用时,Sb(Ⅴ)和S(0)是主要产物,锑释放的表观速率无显著提升,可能与SbOCl和S(0)的生成有关。研究表明,O2能够协同Fe(Ⅲ)氧化Sb2S3,但以Fe(Ⅲ)的作用为主导。本研究揭示了Sb2S3在不同pH值及氧化剂条件下氧化溶解的产物组成特征,提出了不同环境因素影响下的氧化溶解途径,证明碳酸盐岩天然缓冲地层更有利于锑的释放与氧化,岩溶地下水中锑诱发的环境效应会更为严重。Abstract: The stibnite oxidative dissolution under the influence of mining activities is an important process affecting the mobilization of antimony in rock-soil-water environmental medium and its environmental effects. At present, the researches mainly focus on the kinetic characteristic of stibnite dissolution, and the understandings about important issues such as the pathway of stibnite oxidative dissolution, the influence of environmental factors, and the process of antimony release are not clear. In order to study the mechanism of antimony release into groundwater in mining area where the main lithology is carbonatite, important environmental factors pH value and Fe(Ⅲ) were selected. The batch experiment method under single-factor control condition was used to finely describe the oxidation dissolution rate of Sb2S3 and the composition characteristics of Sb and S oxidation products under dark conditions. The results show that Sb2S3 oxidative dissolution was a proton-producing process, and the Sb and S release rates, release pathways and reaction products characteristics were significantly affected by pH value and Fe (Ⅲ). Sb2S3 oxidative dissolution rate changed from rapid to slow and finally reached equilibrium. The magnitude of initial reaction rate was 10-8 mol/(m2·s), and the magnitude of equilibrium reaction rate was 10-10 mol/(m2·s). The release and oxidation rates of antimony increased with the increasing of pH value, and the strong alkali condition was the most favorable condition for the release and oxidation of Sb. Under strong acid condition, the escape of H2S and SO2 gas coupled with the precipitation of elemental sulfur promoted the dissolution of Sb2S3. Sb(Ⅲ) and S(0) were the dominate reaction products. Under neutral condition, dissolved HS- was gradually oxidized to SO42- and a small amount of S2O32-, meanwhile the concentration of Sb(Ⅲ) and Sb(Ⅴ) were similar. Under strong alkali conditions, the formation of SbS33- and Sx- significantly facilitated the Sb2S3 oxidative dissolution rate. Sb(Ⅴ) and S2O32- were the primary species. While Fe(Ⅲ) worked as the lone oxidant, Sb(Ⅴ) and S(0) were the main reaction products. However, the apparent rate of antimony release was not significantly increased, probably due to the precipitation of SbOCl and S(0). Studies have shown that O2 can cooperate with Fe (Ⅲ) to oxidize Sb2S3, meanwhile Fe(Ⅲ) plays the major role in the reaction. This study characterizes the product composition characteristics of Sb2S3 oxidative dissolution under different pH values and oxidants, and proposes the Sb2S3 oxidative dissolution pathways are affected by different environmental factors. Carbonatite formation which can be a natural buffer is conducive to release and oxidation of antimony, and the environmental problem induced by antimony in karst groundwater will be more serious.
-
Key words:
- stibnite /
- pH value /
- Fe(Ⅲ) /
- oxidative dissolution /
- antimony release
-
表 1 不同条件下反应体系的DO浓度和ORP值
Table 1. Dissolved oxygen concentration and oxidation reduction potential of suspension under different conditions
实验组编号 A(仅有O2避光体系) B
(有氧Fe(Ⅲ)避光体系)C
(缺氧Fe(Ⅲ)避光体系)A1 A2 A3 A4 初始pH值 2.02 5.48 6.95 11.42 2 2 DO/(mg·L-1) 7.5~8.7
(8.40±0.40)8.1~9.0
(8.59±0.28)8.1~9.5
(8.71±0.41)8.1~8.8
(8.42±0.21)8.1~9.4
(8.71±0.39)0.7~0.8
(0.760±0.054)ORP/mV 438~448
(445±4.7)238~286
(261±20)203~230
(214±12)112~131
(122±8.8)594~609
(602±6.8)593~611
(602±7.5)表 2 不同氧化剂条件下S(0)和S2O32-的测试结果
Table 2. S(0) and S2O32- concentration in the appearance of different oxidants
反应时间/h O2(A1)1 O2和Fe(Ⅲ)(B) Fe(Ⅲ)(C)1 S(0) S(0) S2O32- S(0) ρB/(mg·L-1) 2 n.a. 5.42 n.a. 3.24 9 n.a. 10.53 n.a. 3.63 30 4.03 9.83 1.99 11.34 60 3.50 12.13 2.57 14.62 96 10.08 13.24 4.94 12.62 144 5.77 9.08 7.27 n.a. 216 5.25 7.61 n.a. n.a. 312 7.21 5.98 n.a. n.a. 432 3.67 7.22 n.a. n.a. 576 n.a. 5.88 n.a. n.a. 注:1A1和C反应体系中均未检出S2O32-;n.a.表示浓度低于检出限 表 3 不同条件下反应体系中锑的平衡反应速率和初始反应速率
Table 3. Antimony equilibrium reaction rate and initial reaction rate under different conditions
实验组 2~576 h
平衡反应速率/(mol·m-2·s-1)0~2 h
初始反应速率/(mol·m-2·s-1)RSbTOT平衡 RSb(Ⅴ)平衡 RSbTOT初始 RSb(Ⅴ)初始 A1 1.8×10-10 1.2×10-10 1.6×10-8 2.8×10-9 A2 2.3×10-10 2.0×10-10 1.4×10-8 2.9×10-9 A3 2.2×10-10 1.8×10-10 1.3×10-8 7.1×10-10 A4 3.0×10-10 2.8×10-10 2.2×10-8 6.6×10-9 B 1.3×10-10 1.3×10-10 6.6×10-9 6.4×10-9 C 9.8×10-11 9.8×10-11 5.9×10-9 5.7×10-9 -
[1] 刘飞, 邓道贵, 祝鹏飞, 等.水环境中不同形态锑的迁移转化及影响因素研究进展[J].安全与环境学报, 2014, 14(2):219-224. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=aqyhjxb201402046 [2] 朱静, 郭建阳, 王立英, 等.锑的环境地球化学研究进展概述[J].地球与环境, 2010, 38(1):109-116. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dzdqhx201001019 [3] He M, Wang X, Wu F, et al.Antimony pollution in China[J].Science of the Total Environment, 2012, 421/422:41-50. doi: 10.1016/j.scitotenv.2011.06.009 [4] Fu Z, Wu F, Mo C, et al.Comparison of arsenic and antimony biogeochemical behavior in water, soil and tailings from Xikuangshan, China[J].Science of the Total Environment, 2016, 539:97-104. doi: 10.1016/j.scitotenv.2015.08.146 [5] 王修, 王建平, 刘冲昊, 等.我国锑资源形势分析及可持续发展策略[J].中国矿业, 2014(5):9-13. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgky201405004 [6] 张许州, 任景玲, 刘宗广, 等.浙闽沿岸海域总溶解态无机锑的分布及影响因素研究[J].环境科学, 2014, 35(2):547-554. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjkx201402021 [7] Mitsunobu S, Takahashi Y, Terada Y, et al.Antimony(Ⅴ) incorporation into synthetic ferrihydrite, goethite, and natural iron oxyhydroxides[J].Environmental Science & Technology, 2010, 44(10):3712-3718. http://www.ncbi.nlm.nih.gov/pubmed/20426473 [8] Li J, Zheng B, He Y, et al.Antimony contamination, consequences and removal techniques:A review[J].Ecotoxicol Environ Saf., 2018, 156:125-134. doi: 10.1016/j.ecoenv.2018.03.024 [9] 吴丰昌, 郑建, 潘响亮, 等.锑的环境生物地球化学循环与效应研究展望[J].地球科学进展, 2008, 23(4):350-356. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dqkxjz200804004 [10] Multani R S, Feldmann T, Demopoulos G P.Antimony in the metallurgical industry:A review of its chemistry and environmental stabilization options[J].Hydrometallurgy, 2016, 164:141-153. doi: 10.1016/j.hydromet.2016.06.014 [11] Rakshit S, Sarkar D, Punamiya P, et al.Antimony sorption at gibbsite-water interface[J].Chemosphere, 2011, 84(4):480-483. doi: 10.1016/j.chemosphere.2011.03.028 [12] 杨森, 谢先军, 肖紫怡, 等.氨基三乙酸对污染土壤中镉活化迁移的影响[J].地质科技情报, 2019, 38(2):243-248. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dzkjqb201902028 [13] 李立刚, 周建伟, 李伟洁, 等.某特大型锑矿区废石中锑的释放规律[J].地质科技情报, 2018, 37(5):215-221. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dzkjqb201805029 [14] 方传棣, 成金华, 赵鹏大, 等.长江经济带矿区土壤重金属污染特征与评价[J].地质科技情报, 2019, 38(5):230-239. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dzkjqb201905024 [15] 武亚遵, 潘春芳, 林云, 等.典型华北型煤矿区主要充水含水层水文地球化学特征及控制因素[J].地质科技情报, 2018, 37(5):191-199. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dzkjqb201805026 [16] He M, Wang N, Long X, et al.Antimony speciation in the environment:Recent advances in understanding the biogeochemical processes and ecological effects[J].Journal of Environmental Sciences, 2019, 75:14-39. doi: 10.1016/j.jes.2018.05.023 [17] Wen B, Zhou A, Zhou J, et al.Coupled S and Sr isotope evidences for elevated arsenic concentrations in groundwater from the world's largest antimony mine, central China[J].Journal of Hydrology, 2017, 557:211-221. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=efa89af6bcfef71bc266b39c382b15fd [18] 莫昌琍, 吴丰昌, 符志友, 等.湖南锡矿山锑矿区农用土壤锑、砷及汞的污染状况初探[J].矿物学报, 2013, 33(3):344-350. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=kwxb201303011 [19] He M, Wang N, Long X, et al.Antimony speciation in the environment:Recent advances in understanding the biogeochemical processes and ecological effects[J].Journal of environmental sciences, 2018, 75:14-39. [20] Zhou J, Nyirenda M T, Xie L, et al.Mine waste acidic potential and distribution of antimony and arsenic in waters of the Xikuangshan Mine, China[J].Applied Geochemistry, 2016, 77:52-61. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dbe4216246db3301647444f03ece11a2 [21] Guo W, Fu Z, Wang H, et al.Environmental geochemical and spatial/temporal behavior of total and speciation of antimony in typical contaminated aquatic environment from Xikuangshan, China[J].Microchemical Journal, 2018, 137:181-189. doi: 10.1016/j.microc.2017.10.010 [22] Wilson S C, Lockwood P V, Ashley P M, et al.The chemistry and behaviour of antimony in the soil environment with comparisons to arsenic:A critical review[J].Environmental Pollution, 2010, 158(5):1169-1181. doi: 10.1016/j.envpol.2009.10.045 [23] 王华伟, 李晓月, 李卫华, 等.pH和络合剂对五价锑在水钠锰矿和水铁矿表面吸附行为的影响[J].环境科学, 2017, 38(1):180-187. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjkx201701021 [24] Gleisner M, Herbert R B, Kockum P C F.Pyrite oxidation by Acidithiobacillus ferrooxidans at various concentrations of dissolved oxygen[J].Chemical Geology, 2006, 225(1/2):16-29. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=4287a7433d6d1aaad951e1cdfab3de3b [25] Biver M, Shotyk W.Stibnite(Sb2S3) oxidative dissolution kinetics from pH 1 to 11[J].Geochimica et Cosmochimica Acta, 2012, 79:127-139. doi: 10.1016/j.gca.2011.11.033 [26] Hu X, He M, Kong L.Photopromoted oxidative dissolution of stibnite[J].Applied Geochemistry, 2015, 61:53-61. doi: 10.1016/j.apgeochem.2015.05.014 [27] Herath I, Vithanage M, Bundschuh J.Antimony as a global dilemma:Geochemistry, mobility, fate and transport[J].Environmental Pollution, 2017, 223:545-559. doi: 10.1016/j.envpol.2017.01.057 [28] Morse J W, Millero F J, Cornwell J C, et al.The chemistry of the hydrogen sulfide and iron sulfide systems in natural waters[J].Earth-Science Reviews, 1987, 24(1):1-42. doi: 10.1016/0012-8252(87)90046-8 [29] Leuz A K, Johnson C A.Oxidation of Sb(Ⅲ) to Sb(Ⅴ) by O2 and H2O2 in aqueous solutions[J].Geochimica et Cosmochimica Acta, 2005, 69(5):1165-1172. doi: 10.1016/j.gca.2004.08.019 [30] Borilova S, Mandl M, Zeman J, et al.Can sulfate be the first dominant aqueous sulfur species formed in the oxidation of pyrite by Acidithiobacillus ferrooxidans[J].Frontiers in Microbiology, 2018, 9:3134. doi: 10.3389/fmicb.2018.03134 [31] Raschman P, Emília S.Kinetics of leaching of stibnite by mixed Na2S and NaOH solutions[J].Hydrometallurgy, 2012, 113:60-66. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=d20ca2c235535f099c25485aa9d4729e [32] Gök Ö.Catalytic production of antimonate through alkaline leaching of stibnite concentrate[J].Hydrometallurgy, 2014, 149:23-30. doi: 10.1016/j.hydromet.2014.06.007 [33] Kamyshny A, Borkenstein C G, Ferdelman T G.Protocol forquantitative detection of elemental sulfur and polysulfide zero-valent sulfur distribution in natural aquatic samples[J].Geostandards and Geoanalytical Research, 2009, 33(3):415-435. doi: 10.1111/j.1751-908X.2009.00907.x [34] 温冰.湖南锡矿山水环境中锑来源及迁移转化的多元同位素解析[D].武汉: 中国地质大学(武汉), 2017. -





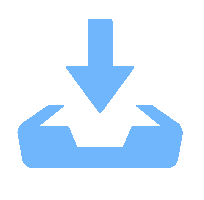 下载:
下载: