Source and enrichment mechanism of ammonium in shallow confined aquifer in the west of Dongting Plain
-
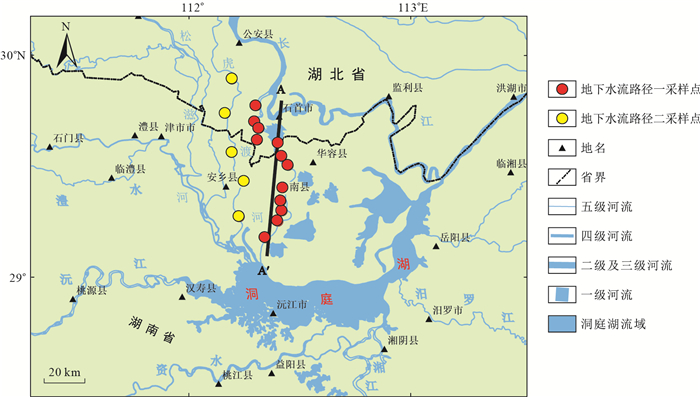
摘要: 洞庭湖平原西部地区浅层承压含水层是当地主要的地下水开采层,却面临严重的水质型缺水问题,其中以铵氮异常最为典型,但目前对于其来源和富集机制的认识十分薄弱。以洞庭湖平原西部为研究区,沿区域地下水流方向对地下水样品进行水文地球化学分析,旨在查明地下水中铵氮的来源,揭示地下水流动对铵氮富集的控制机理。结果表明:NH4-N质量浓度为0.05~16.75 mg/L,且与DOC、HCO3-、As、Fe2+、Mn、P质量浓度呈现较好正相关性;而高质量浓度的NH4-N对应着很低质量浓度的Cl-、SO42-、NO3-和很低的Cl/Br比值,可以推测浅层承压水中的铵氮主要由天然有机质矿化作用产生,而非人为输入。沿着地下水流向,NH4-N和As、Fe2+、Mn质量浓度均显著升高,说明由于水流越来越滞缓,含水介质颗粒越来越细,沉积物有机质越来越富集,含氮有机质矿化作用逐渐增强,使得NH4-N质量浓度逐渐升高,并形成了还原性逐渐增强的地下水环境,相关地球化学过程产生的还原性组分(砷、铁、锰等)也逐渐富集。本研究进一步丰富了地下水原生铵氮的成因理论,可为当地的供水安全保障提供理论基础。Abstract: Shallow confined aquifer is the main groundwater exploitation layer in the West of Dongting Plain, but it is faced with serious water shortage owing to worse water quality, among which ammonium anomaly is the most typical.However, its source and enrichment mechanism has been poorly understood at present.Taking the west of Dongting Plain as the study area, the hydrogeochemical analysis of groundwater samples along the direction of regional groundwater flow was carried out to find out the source of ammonium in groundwater and reveal the controlling mechanism of groundwater flow to the enrichment of ammonium.The results showed that the concentration of NH4-N was 0.05~16.75 mg/L, and had good positive correlations with DOC, HCO3-, As, Fe2+, Mn and P, while the high concentration of NH4-N corresponded to very low concentrations of Cl-, SO42-, NO3-, and very low Cl/Br ratio, it can be speculated that ammonium in shallow confined aquifer was produced by the mineralization of natural organic matter rather than anthropogenic input.Along the groundwater flow direction, the concentrations of NH4-N, As, Fe2+ and Mn increased significantly, indicating that with the more sluggsih groundwater flowed, the particles of water-bearing media were becoming finer and finer, and the organic matter in sediments was more and more enriched, thus the mineralization of nitrogen-bearing organic matter was gradually enhanced and the concentration of NH4-N increased gradually, forming a gradually reduced groundwater environment.As a result, the reductive components (arsenic, iron, manganese, etc) produced from related geochemical processes were also gradually enriched.The study further enriches the genetic theory of geogenic ammonium in groundwater and provides theoretical basis for the safety and security of local water supply.
-
图 2 研究区水文地质平面图(a)和水文地质剖面图(b) (据文献[19]修改)
Figure 2. Hydrogeological plan (a), and hydrogeological profile (b) in the study area
表 1 洞庭湖平原第四系地层岩性
Table 1. Lithology Quaternary strata in the Dongting Plain
地层代号 地层名称 埋藏深度/m 主要岩性 水文地质特征 Qh dal 洞庭湖组河湖相沉积 20~25 灰色、灰褐色砂质黏土、含钙质黏土 浅层孔隙潜水含水岩组由第四系全新统组成,广泛分布于湖区平原及湘江、资江、沅江、澧水4条水系的河漫滩上,厚度为5~20 m Qh dl 洞庭湖组湖沼相沉积 50~55 灰色、灰褐色、灰黑色、棕褐色砂质黏土,粉砂、细砂黏土,含钙质粉砂质黏土和淤泥。底部偶含细砾 浅层孔隙承压含水层由上更新统、中更新统组成,厚度为54~150 m。含水量大,易于开采,是区内开采量最大的含水层 Qp3 xs 下蜀组 55~60 黄褐色、棕黄色、灰黄色含铁锰质结核和薄膜的砂质黏土、粉砂质黏土、黏土、粉砂,局部含淤泥质和炭化植物残骸,底部偶发育细砾层 Qp2 mw 马王堆组 130~150 砂砾层、含砾砂层,偶夹黏土 Qp2 b 白沙井组 200~210 冲湖积形成的棕黄色、棕红色砾层、砂层及黏土层 深层孔隙-裂隙承压含水岩组在区内分布广泛,含水介质在水平方向和垂向上都存在很大差异 Qp2 x 新开铺组 280~290 河湖交互相砾石层与黄色砂砾层、粗砾层及泥砾砂互层 Qp1 m 汨罗组 400~410 河湖交互相碎屑黏土 表 2 地下水水化学指标统计
Table 2. Statistics of groundwater hydrochemical indexes
水化指标 18S-25G 18S-26G 18S-27G 18S-28G 18S-29G 18S-30G 18S-31G 18S-32G 18S-33G 18S-34G 18S-35G 18S-36G 18S-39G 18S-40G 18S-41G 18S-42G 18S-43G 最大值 最小值 平均值 变异系数 pH 7.32 7.70 7.33 7.16 7.66 7.26 7.32 7.08 7.15 7.19 6.83 6.66 6.76 7.13 7.24 7.31 7.52 7.70 6.66 7.21 0.04 EC/(μS·cm-1) 435 453 492 396 406 407 481 445 440 508 637 600 759 582 396 399 458 759.00 396.00 487.88 0.21 Eh/mV -88.1 -54.9 -73.4 -25.0 -47.4 -79.3 -73.1 -88.0 -73.6 -90.5 -104 -84.7 -96.4 -88.2 -50.4 -72.1 -42.4 -25.00 -104 -72.40 -0.30 NH4-N 0.20 0.31 0.12 0.05 0.14 0.07 1.07 0.75 0.65 2.25 16.75 11.10 7.50 0.70 0.40 0.14 0.14 16.75 0.05 2.49 1.91 Fe2+ 2.25 0.72 0.77 0.58 0.61 0.83 1.57 1.95 1.70 5.45 11.10 20.20 9.70 1.60 2.05 0.74 0.74 20.20 0.58 3.68 1.44 Ca2+ 64.60 50.60 59.10 42.60 57.90 67.80 69.80 61.70 64.50 64.40 77.40 70.40 102 62.90 51.70 59.20 67.80 102 42.60 64.40 0.20 Mg2+ 14.10 18.50 18.40 14.80 15.90 12.10 19.70 18.70 16.30 22.20 21.20 25.60 37.00 24.00 15.90 14.50 18.70 37.00 12.10 19.30 0.30 Na+ 34.90 47.60 51.50 38.10 23.90 26.30 32.40 32.80 28.90 35.30 20.60 16.50 35.60 47.60 35.70 32.80 34.60 51.50 16.50 33.80 0.27 K+ 0.20 0.32 0.21 0.33 0.50 0.28 0.16 0.15 0.26 0.27 0.74 0.61 0.73 0.21 0.15 0.43 0.34 0.74 0.15 0.35 0.56 Cl- 35.90 1.12 3.86 2.19 4.20 1.37 0.68 0.86 1.29 1.30 3.93 0.80 1.09 3.42 0.81 0.76 0.72 35.90 0.72 3.80 2.21 NO3- ρB/(mg·L-1) 0.58 0.55 0.55 0 1.03 0.56 0.55 0.57 0.54 0.56 0.56 0.60 0.59 1.03 0.60 0 0.59 1.03 0 0.53 0.43 SO42- 2.72 0 1.32 2.31 3.45 1.06 0 0 0 0 0 0 0 0 0 0.95 1.01 3.45 0 0.75 1.47 HCO3- 277 366 410 314 321 332 389 356 347 399 460 454 617 425 320 333 377 617 277 382.00 0.21 DOC 2.91 1.84 3.32 6.78 2.52 2.09 5.47 4.15 2.71 1.58 6.58 5.22 5.15 1.35 1.58 1.11 1.39 6.78 1.11 3.28 0.58 Fe 1.01 0.39 0.76 0.37 0.20 0.80 1.47 2.03 1.72 4.86 15.90 20.80 11.10 1.57 0.85 0.90 0.33 20.80 0.20 3.83 1.60 Mn 0.49 0.09 0.30 0.15 0.91 0.32 0.26 0.21 0.17 0.33 0.36 1.73 0.68 0.30 0.24 0.13 0.10 1.73 0.09 0.40 1.02 P 0.22 0.07 0.06 0.12 0.10 0.09 0.53 0.74 0.51 1.27 3.45 1.02 2.17 0.86 0.26 0.06 0.05 3.45 0.06 0.68 1.34 As ρB/(μg·L-1) 1.47 0.72 2.34 0.27 4.56 1.01 19.60 8.87 2.33 3.65 26.60 33.20 9.33 3.13 2.62 0.73 0.79 33.20 0.27 7.13 1.39 S2- 13.00 3.00 0 4.00 2.00 10 5.00 7.00 3.00 9.00 19.00 0 22.00 2.00 13.00 13.00 13.00 22.00 0 8.12 0.81 井深/m 36 30 30 40 58 35 40 25 53 31 38 30 40 25 30 19 40 58 19 35.29 0.04 表 3 世界范围内已报道的天然高铵氮地下水
Table 3. Natural high concentration of ammonium groundwater reported worldwide
地点 深度/m 最高铵氮质量浓度/(mg·L-1) 水文地质背景 来源 中国珠江三角洲 20~50 390 海岸带更新世砂质含水层 文献[10] 意大利费拉拉 8~10 45 海岸带泛滥平原,第四系古沼泽相泥炭层 文献[11] 越南红河三角洲 50~90 100 河口三角洲平原,第四系泥炭层 文献[12] 孟加拉盆地 28~45 约23 全新世三角洲平原下伏含水层 文献[25] 中国江汉平原 20~30 14.1 冲湖积平原,第四系松散沉积物 文献[27] 美国密歇根 16~19 13.5 冰碛相含水层,全新世湖相淤泥层 文献[29] 澳大利亚珀斯 约20 17.6 潮汐河口第四系砂-黏土夹层 文献[30] 墨西哥北部 < 20 19.2 第四系弱透水层富黏土,咸水 文献[31] -
[1] 陈新明, 马腾, 蔡鹤生, 等.地下水氮污染的区域性调控策略[J].地质科技情报, 2013, 32(6):130-143, 149. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dzkjqb201306020 [2] Pacheco F A L, Sanches L F.Environmental land use conflicts in catchments: A major cause of amplified nitrate in river water[J].Science of the Total Environment, 2016, 548/549:173-188. http://www.ncbi.nlm.nih.gov/pubmed/26802346 [3] Nikolenko O, Jurado A, Alberto V, et al.Isotopic composition of nitrogen species in groundwater under agriculture areas:A review[J].Science of the Total Environment, 2018, 621:1342-1415. http://www.ncbi.nlm.nih.gov/pubmed/29074237 [4] 宁卓, 张翠云, 张胜.地下水铵污染及其氮同位素研究[J].南水北调与水利科技, 2011, 9(3):129-132. http://www.cnki.com.cn/Article/CJFDTotal-NSBD201103033.htm [5] 章颖, 王锦国.奎河铜山段两岸浅层地下水铵氮污染特征研究[J].中国煤炭地质, 2017, 29(8):60-66, 77. http://d.wanfangdata.com.cn/Periodical/zgmtdz201708011 [6] 吕晓立, 韩占涛, 张海岭, 等.某典型化肥厂污染场地地下水中铵氮污染特征及成因[J].干旱区资源与环境, 2018, 32(12):176-182. http://www.cqvip.com/QK/96735X/201812/676633914.html [7] World Health Organization.Guidelines for drinking water Quality[S].Geneva: WHO, 1993. [8] Buss S R, Herbert A W, Morgan P, et al.A review of ammonium attenuation in soil and groundwater[J].Quarterly Journal of Engineering Geology and Hydrogeology, 2004, 37:347-359. http://www.researchgate.net/publication/301518618_Review_of_ammonium_attenuation_in_soil_and_groundwater [9] Huang X P, Huang L M, Yue W Z.The characteristics of nutrients and eutrophication in the Pearl River estuary, South China[J].Marine Pollution Bulletin, 2003, 47(1/6):30-36. http://www.ncbi.nlm.nih.gov/pubmed/12787594 [10] Jiao J J, Wang Y, Cherry J A, et al.Abnormally high ammonium of natural origin in a coastal aquifer-aquitard system in the Pearl River Delta, China[J].Environmental Science & Technology, 2010, 44(19):7470-7475. [11] Mastrocicco M, Giambastiani B M S, Colombani N.Ammonium occurrence in a salinized lowland coastal aquifer (Ferrara, Italy)[J].Hydrological Processes, 2013, 27(24):3495-3501. [12] Norrman J, Sparrenbom C J, Berg M, et al.Tracing sources of ammonium in reducing groundwater in a well eld in Hanoi (Vietnam) by means of stable nitrogen isotope (δ15N) values[J].Applied Geochemistry, 2015, 61:248-258. [13] 胡光伟, 张明, 刘珍, 等.洞庭湖水质变化及其形成机制分析[J].水资源与水工程学报, 2019, 30(3):39-45. http://www.cnki.com.cn/Article/CJFDTotal-XBSZ201903006.htm [14] 刘长明, 皮建高, 肖江.洞庭湖区浅层地下水环境质量综合分区评价[J].今日科苑, 2009(15):155-156. http://www.cnki.com.cn/Article/CJFDTotal-JRKR200915124.htm [15] 王璨, 姚腾飞, 陈亮晶, 等.江汉-洞庭平原地下水资源及其环境问题调查评价(湖南)报告[R].长沙: 湖南省地质调查院, 2016. [16] 覃红燕.近50余年洞庭湖水文环境演变及其成因分析[D].长沙: 湖南农业大学, 2013. [17] 连生土.洞庭湖区浅层地下水环境质量评价研究[D].湖南湘潭: 湖南科技大学, 2011. [18] 王军霞.江汉-洞庭平原流域水文模型与地下水数值模型耦合模拟研究[D].武汉: 中国地质大学(武汉), 2015. [19] 中国地质调查局武汉地质调查中心.江汉-洞庭平原地下水资源及其环境问题调查评价第四纪地质图(1: 25万)[R].武汉: 中国地质调查局武汉地质调查中心, 2015. [20] 刘伟江, 袁祥美, 张雅, 等.贵阳市岩溶地下水水化学特征及演化过程分析[J].地质科技情报, 2018, 37(6):245-251. http://www.cnki.com.cn/Article/CJFDTotal-DZKQ201806031.htm [21] 郭清海, 王焰新.水文地球化学信息对岩溶地下水流动系统特征的指示意义:以山西神头泉域为例[J].地质科技情报, 2006, 25(3):85-88. http://www.cnki.com.cn/Article/CJFDTotal-DZKQ200603015.htm [22] 沈帅, 马腾, 杜尧, 等.江汉平原东部浅层地下水氮的空间分布特征[J].环境科学与技术, 2018, 41(2):47-56. http://www.cnki.com.cn/Article/CJFDTotal-FJKS201802008.htm [23] McArthur J M, Sikdar P K, Hoque M A, et al.Waste-water impacts on groundwater:Cl/Br ratios and implications for arsenic pollution of groundwater in the Bengal Basin and Red River Basin, Vietnam[J].Science of the Total Environment, 2012, 437:390-402. [24] 邬建勋, 余倩, 蒋庆肯, 等.江汉平原高砷地下水与含水层沉积物的地球化学特征[J].地质科技情报, 2019, 38(1):250-257. http://www.cqvip.com/QK/93477A/20191/68907581504849574849485056.html [25] McArthur J M, Ravenscroft P, Safiulla S, et al.Arsenic in groundwater:Testing pollution mechanisms for sedimentary aquifers in Bangladesh[J].Water Resources Research, 2001, 37(1):109-117. doi: 10.1029/2000WR900270/full [26] 王佳琪, 马瑞, 孙自永.地表水与地下水相互作用带中氮素污染物的反应迁移机理及模型研究进展[J].地质科技情报, 2019, 38(4):270-280. http://www.cnki.com.cn/Article/CJFDTotal-DZKQ201904029.htm [27] Du Y, Ma T, Deng Y M, et al.Sources and fate of high levels of ammonium in surface water and shallow groundwater of the Jianghan Plain, Central China[J].Environmental Science:Processes & Impacts, 2017, 19:161-172. [28] Li X, Tang C Y, Cao Y J, et al.Carbon, nitrogen and sulfur isotopic features and the associated geochemical processes in a coastal aquifer system of the Pearl River Delta, China[J].Journal of Hydrology, 2019, 575:986-998. [29] Lingle D A, Kehew A E, Krishnamurthy R V.Use of nitrogen isotopes and other geochemical tools to evaluate the source of ammonium in a confined glacial drift aquifer, Ottawa County, Michigan, USA[J].Applied Geochemistry, 2017, 78:334-342. http://www.sciencedirect.com/science/article/pii/S0883292717300124 [30] Linderfelt W R, Turner J V.Interaction between shallow groundwater, saline surface water and nutrient discharge in a seasonal estuary: The Swan-Canning system[J].Hydrological Processes, 2001, 15(13):2631-2653. [31] Ortega-Guerrero A.Origin and geochemical evolution of groundwater in a closed-basin clayey aquitard, Northern Mexico[J].Journal of Hydrology, 2003, 284(1):26-44. [32] Du Y, Ma T, Deng Y M, et al.Hydrogeochemical evidences for targeting sources of safe groundwater supply in arsenic-affected multi-level aquifer systems[J].The Science of the Total Environment, 2018, 645:1159-1171. -





 下载:
下载: