Research progress of fluid-granite interaction in CO2 based enhanced geothermal system
-
摘要: CO2与水相比,膨胀性大、黏度低、与岩石反应程度低,并且在作为增强型地热系统(EGS)渗流传热流体时,比水具有更高的换热效率。对CO2-EGS生产过程中储层岩石物性变化的研究具有重要意义,从理论研究、实验研究、数值模拟3个方面,对CO2基增强型地热系统CO2-EGS中流体-岩石相互作用的研究现状进行了总结,并且从矿物成分、微观孔结构和力学性质3个方面对储层岩石性质的变化进行了评价。结果表明,CO2-水-岩石相互作用参与反应的矿物主要为石英、长石类;而沉淀的矿物为蒙脱石、伊利石及方解石等。CO2-水-岩石相互作用会导致储层岩石的力学性质下降,孔隙结构特征改变。最后,讨论了CO2作为EGS渗流传热流体仍需攻克的难点问题,包括:CO2的热动力学特征、换热效率,CO2-水-岩石的相互作用及其对储层性质的改变,影响CO2-EGS经济性的因素,以及CO2-EGS数值模型的研究等。针对这些方面的研究可为今后CO2-EGS的开发奠定基础。
-
关键词:
- CO2基增强型地热系统(CO2-EGS) /
- 花岗岩 /
- 化学作用 /
- 实验研究 /
- 数值模拟
Abstract: Compared with water, CO2 has larger expansibility, lower viscosity and lower reaction degree with rock.As an enhanced geothermal system (EGS) seepage heat transfer fluid, CO2 has higher heat transfer efficiency than water.This paper summarizes the research status of fluid/rock interaction in CO2-EGS from three aspects: theoretical research, experimental research and numerical simulation.The changes of reservoir rock properties are evaluated from mineral composition, micro pore structure and mechanical properties.The results show that the main reaction minerals of CO2-water-rock interaction are quartz and feldspar, and the precipitation minerals are montmorillonite, illite and calcite.The interaction of CO2-water-rock will lead to the decline of mechanical properties of reservoir rocks and the change of pore structure characteristics.Finally, the existing problems and development trend of CO2-water-rock interaction in CO2-EGS are discussed, which provides a reference for the development and research of CO2-EGS in the future. -
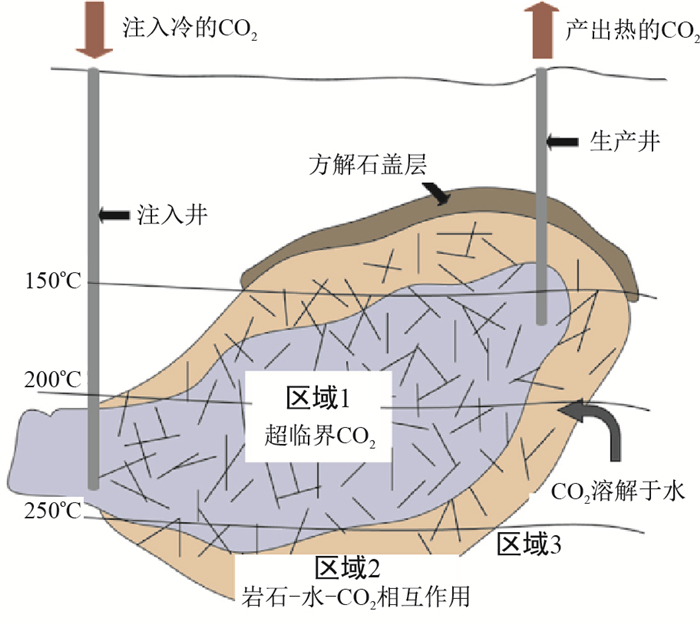
图 1 CO2-EGS储层区域划分示意图(据文献[24])
Figure 1. Schematic diagram of regional division of CO2-EGS reservoir
-
[1] 许天福, 胡子旭, 李胜涛, 等. 增强型地热系统: 国际研究进展与我国研究现状[J]. 地质学报, 2018, 92(9): 1936-1947. doi: 10.3969/j.issn.0001-5717.2018.09.012Xu T F, Hu Z X, Li S T, et al. Enhanced geothermal system: International progresses and research status of China[J]. Acta Geologica Sinica, 2018, 92(9): 1936-1947(in Chinese with English abstract). doi: 10.3969/j.issn.0001-5717.2018.09.012 [2] 窦斌, 高辉, 周刚, 等. 我国发展增强型地热开采技术所面临的机遇与挑战[J]. 地质科技情报, 2014, 33(5): 208-210. https://www.cnki.com.cn/Article/CJFDTOTAL-DZKQ201405032.htmDou B, Gao H, Zhou G, et al. Opportunities and challenges of developing enhance geothermal system technology in China[J]. Geological Science and Technology Information, 2014, 33(5): 208-210(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-DZKQ201405032.htm [3] 肖鹏, 窦斌, 田红, 等. 开采海洋区域干热岩的可行性探讨[J]. 海洋地质前沿, 2018, 34(8): 55-60. https://www.cnki.com.cn/Article/CJFDTOTAL-HYDT201808007.htmXiao P, Dou B, Tian H, et al. Feasibility of exploitation of submarine hot dry rock in offshore area[J]. Marine Geology Frontiers, 2018, 34(8): 55-60(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-HYDT201808007.htm [4] 徐超, 窦斌, 田红, 等. 二氧化碳爆破致裂建造增强型地热系统热储层工艺探讨[J]. 地质科技情报, 2019, 38(5): 247-252. https://www.cnki.com.cn/Article/CJFDTOTAL-DZKQ201905027.htmXu C, Dou B, Tian H, et al. Process of carbon dioxide blasting to build EGS thermal reservoir[J]. Geological Science and Technology Information, 2019, 38(5): 247-252(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-DZKQ201905027.htm [5] 荆铁亚, 赵文韬, 郜时旺, 等. 干热岩地热开发实践及技术可行性研究[J]. 中外能源, 2018, 23(11): 17-22. https://www.cnki.com.cn/Article/CJFDTOTAL-SYZW201811004.htmJing T Y, Zhao W T, Gao S W, et al. Practice and technical feasibility study of hot dry rock geothermal development[J]. Sino-Global Energy, 2018, 23(11): 17-22(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-SYZW201811004.htm [6] 康玲, 王时龙, 李川. 增强地热系统EGS的人工热储技术[J]. 机械设计与制造, 2008(9): 141-143. doi: 10.3969/j.issn.1001-3997.2008.09.059Kang L, Wang S L, Li C. Reservoir technology in enhanced geothermal systems[J]. Machinery Design & Manufacture, 2008(9): 141-143(in Chinese with English abstract). doi: 10.3969/j.issn.1001-3997.2008.09.059 [7] 国家能源局. 地热能术语[S]. 北京: 中国石化出版社, 2018.National Energy Administration. Geothermal energy terminology[S]. Beijing: Sinopec Press, 2018(in Chinese). [8] Panel M L. The future of geothermal energy. Impact of enhanced geothermal systems[EGS] on the United States in the 21st century[J]. Geothermics, 2006, 17(5/6): 881-882. http://www.osti.gov/scitech/biblio/1220063 [9] Fard M H, Hooman K, Chua H T. Numerical simulation of a supercritical CO2 geothermosiphon[J]. International Communications in Heat & Mass Transfer, 2010, 37(10): 1447-1451. http://www.sciencedirect.com/science/article/pii/S0735193310002150 [10] Na J, Xu T, Yuan Y, et al. An Integrated Study of Fluid-Rock Interaction in a CO2-based Enhanced Geothermal System: A Case Study of Songliao Basin, China[J]. Applied Geochemistry, 2015, 59: 166-177. doi: 10.1016/j.apgeochem.2015.04.018 [11] 许天福, 张延军, 曾昭发, 等. 增强型地热系统(干热岩)开发技术进展[J]. 科技导报, 2012, 30(32): 42-45. doi: 10.3981/j.issn.1000-7857.2012.32.004Xu T F, Zhang Y J, Zeng Z F, et al. Technology progress in an enhanced geothermal system (hot dry rock)[J]. Science & Technology Review, 2012, 30(32): 42-45(in Chinese with English abstract). doi: 10.3981/j.issn.1000-7857.2012.32.004 [12] Brown D A Hot Dry Rock geothermal energy concept utilizing supercritical CO2 instead of water[C]. Stanford, California: Proceedings of the Twenty-Fifth Workshop on Geothermal Reservoir Engineering, Stanford University, 2000. [13] Atrens A D, Gurgenci H, Rudolph V. Economic optimization of a CO2-based EGS power plant[J]. Energy & Fuels, 2011, 25(8): 3765-3775. doi: 10.1021/ef200537n [14] Zhang F Z, Jiang P X, Xu R N. System thermodynamic performance comparison of CO2-EGS and water-EGS systems[J]. Applied Thermal Engineering, 2013, 61(2): 236-244. doi: 10.1016/j.applthermaleng.2013.08.007 [15] Chu S, Majumdar A. Opportunities and challenges for a sustainable energy future[J]. Nature, 2012, 488(7411): 294-303. doi: 10.1038/nature11475 [16] Pruess K. On production behavior of enhanced geothermal systems with CO2 as working fluid[J]. Energy Conversion & Management, 2008, 49(6): 1446-1454. http://www.sciencedirect.com/science/article/pii/S019689040800006X [17] Pruess K. Enhanced geothermal systems (EGS) using CO2 as working fluid-A novel approach for generating renewable energy with simultaneous sequestration of carbon[J]. Geothermics, 2006, 35(4): 351-367. doi: 10.1016/j.geothermics.2006.08.002 [18] Cui G, Ren S, Rui Z, et al. The influence of complicated fluid-rock interactions on the geothermal exploitation in the CO2, plume geothermal system[J]. Applied Energy, 2017, 227: 49-63. http://www.sciencedirect.com/science/article/pii/S0306261917315593 [19] Wu Y, Li P. The potential of coupled carbon storage and geothermal extraction in a CO2-enhanced geothermal system: a review[J]. Geothermal Energy, 2020, 8(1): 1-28. doi: 10.1186/s40517-020-0157-0 [20] Sun F, Yao Y, Li G, et al. Geothermal energy development by circulating CO2 in a U-shaped closed loop geothermal system[J]. Energy Conversion and Management, 2018, 174: 971-982. doi: 10.1016/j.enconman.2018.08.094 [21] Pan L, Freifeld B, Doughty C, et al. Fully coupled wellbore-reservoir modeling of geothermal heat extraction using CO2 as the working fluid[J]. Geothermics, 2015, 53: 100-113. doi: 10.1016/j.geothermics.2014.05.005 [22] Luo F, Xu R N, Jiang P X. Numerical investigation of fluid flow and heat transfer in a doublet enhanced geothermal system with CO2 as the working fluid (CO2-EGS)[J]. Energy, 2014, 64: 307-322. doi: 10.1016/j.energy.2013.10.048 [23] Bataillé A, Genthon P, Rabinowic Z M, et al. Modeling the coupling between free and forced convection in a vertical permeable slot: Implications for the heat production of an Enhanced Geothermal System[J]. Geothermics, 2010, 35(5): 654-682. [24] Ueda A, Kato K, Ohsumi T, et al. Experimental studies of CO2-rock interaction at elevated temperatures under hydrothermal conditions[J]. Geochemical Journal, 2005, 39(5): 417-425. doi: 10.2343/geochemj.39.417 [25] Isaka B L A, Ranjith P G, Rathnaweera T D, et al. Influence of long-term operation of supercritical carbon dioxide based enhanced geothermal system on mineralogical and microstructurally-induced mechanical alteration of surrounding rock mass[J]. Renewable energy, 2019, 136: 428-441. doi: 10.1016/j.renene.2018.12.104 [26] Giolito C, Ruggieri G, Gianelli G. Fluid evolution in the deep reservoir of the Mt Amiata geothermal field, Italy[J]. Transactions - Geothermal Resources Council, 2007, 31: 153-158. [27] 侯大力, 罗平亚, 王长权, 等. 高温高压下CO2在水中溶解度实验及理论模型[J]. 吉林大学学报: 地球科学版, 2015, 45(2): 564-572. https://www.cnki.com.cn/Article/CJFDTOTAL-CCDZ201502026.htmHou D L, Luo P Y, Wang C Q, et al. Experimental research and theoretical model for CO2 solubility in water under high temperature and high pressure[J]. Journal of Jilin University (Earth Science Edition), 2015, 45(2): 564-572(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-CCDZ201502026.htm [28] 李靖. 高温高压高含CO2天然气在地层水中溶解度理论研究[D]. 成都: 西南石油大学, 2017.Li J. Theoretical study on solubility of high CO2 content natural gas in formation water at high temperature and high pressure high[D]. Chengdu: Southwest Petroleum University, 2017(in Chinese with English abstract). [29] 陈东灿, 窦斌, 田红, 等. 基于花岗闪长岩矿物成分的热导率预测模型[J]. 地质科技情报, 2019, 38(2): 262-266. https://www.cnki.com.cn/Article/CJFDTOTAL-DZKQ201902031.htmChen D C, Dou B, Tian H, et al. Thermal conductivity prediction model based on mineral composition of granodiorite[J]. Geological Science and Technology Information, 2019, 38(2): 262-266(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-DZKQ201902031.htm [30] 喻勇, 徐达, 窦斌, 等. 高温花岗岩遇水冷却后可钻性试验研究[J]. 地质科技情报, 2019, 38(4): 287-292. https://www.cnki.com.cn/Article/CJFDTOTAL-DZKQ201904031.htmYu Y, Xu D, Dou B, et al. Experimental study on drillability of high temperature granite after water cooling[J]. Geological Science and Technology Information, 2019, 38(4): 287-292(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-DZKQ201904031.htm [31] Suto Y, Liu L, Yamasaki N, et al. Initial behavior of granite in response to injection of CO2-saturated fluid[J]. Applied Geochemistry, 2007, 22(1): 202-218. doi: 10.1016/j.apgeochem.2006.09.005 [32] Lin H, Fujii T, Takisawa R, et al. Experimental evaluation of interactions in supercritical CO2/water/rock minerals system under geologic CO2 sequestration conditions[J]. Journal of Materials science, 2008, 43(7): 2307-2315. doi: 10.1007/s10853-007-2029-4 [33] Liu L, Suto Y, Bignall G, et al. CO2 injection to granite and sandstone in experimental rock/hot water systems[J]. Energy Conversion & Management, 2003, 44(9): 1399-1410. http://www.ingentaconnect.com/content/el/01968904/2003/00000044/00000009/art00002 [34] Lo Ré C, Kaszuba J P, Moore J N, et al. Fluid-rock interactions in CO2-saturated, granite-hosted geothermal systems: Implications for natural and engineered systems from geochemical experiments and models[J]. Geochimica et Cosmochimica Acta, 2014, 141: 160-178. doi: 10.1016/j.gca.2014.06.015 [35] Shiraki R, Dunn T L. Experimental study on water-rock interactions during CO2 flooding in the Tensleep Formation, Wyoming, USA[J]. Applied Geochemistry, 2000, 15(3): 265-279. doi: 10.1016/S0883-2927(99)00048-7 [36] Watson M N, Zwingmann N, Lemon N M. The Ladbroke Grove-Katnook carbon dioxide natural laboratory: A recent CO2 accumulation in a lithic sandstone reservoir[J]. Energy, 2004, 29(9/10): 1457-1466. http://www.sciencedirect.com/science/article/pii/S0360544204001628 [37] Rosenbauer R J, Koksalan T, Palandri J L. Experimental investigation of CO2-brine-rock interactions at elevated temperature and pressure: Implications for CO2 sequestration in deep-saline aquifers[J]. Fuel Processing Technology, 2005, 86(14/15): 1581-1597. http://www.sciencedirect.com/science/article/pii/S037838200500024X [38] Sugama T, Ecker L, Butcher T. Carbonation of rock minerals by supercritical carbon dioxide at 250℃[M]. Upton, NY, USA: Energy Science & Technology Department, Brookhaven National Laboratory, 2010. [39] Sugama T, Gill S, Ecker L, et al. Susceptibility of granite rock to ScCO2/water at 200℃ and 250℃[M]. Upton NY USA: Energy Science & Technology Department, Brookhaven National Laboratory, 2011. [40] Lo Ré C, Kaszuba J, Moore J, et al. Supercritical CO2 in a granite-hosted geothermal system: experimental insights into multiphase fluid-rock interactions[C]. Stanford, California: The thirty-seventh workshop on geothermal reservoir engineering Proceedings, Stanford University, 2012. [41] Yoo S Y, Kuroda Y, Mito Y, et al. A geochemical clogging model with carbonate precipitation rates under hydrothermal conditions[J]. Applied Geochemistry, 2013, 30(2): 67-74. http://www.sciencedirect.com/science/article/pii/S0883292712002090 [42] Kaieda H, Ueda A, Kubota K, et al. Field experiments for studying on CO2 sequestration in solid minerals at the Ogachi HDR geothermal site, Japan[C]. Stanford, California: Thirty-fourth Workshop on Geothermal Reservoir Engineering, Stanford University, 2009. [43] Jung Y, Xu T, Dobson P F, et al. Experiment-based modeling of geochemical interactions in CO2-based geothermal systems[C]. Stanford, California: The thirty-eighet workshop on geothermal reservoir engineering Proceedings, Stanford University, 2013. [44] Borgia A, Pruess K, Kneafsey T J, et al. Numerical simulation of salt precipitation in the fractures of a CO2 enhanced geothermal system[J]. Geothermics, 2012, 44: 13-22. doi: 10.1016/j.geothermics.2012.06.002 [45] Johnson J W, Nitao J J, Knauss K G. Reactive transport modelling of CO2 storage in saline aquifers to elucidate fundamental processes, trapping mechanisms and sequestration partitioning[J]. Geological Society London Special Publications, 2004, 233(1): 107-128. doi: 10.1144/GSL.SP.2004.233.01.08 [46] Ketzer J M, Iglesias R, Einloft S, et al. Water-rock-CO2 interactions in saline aquifers aimed for carbon dioxide storage: Experimental and numerical modeling studies of the Rio Bonito Formation (Permian), southern Brazil[J]. Applied Geochemistry, 2009, 24(5): 760-767. doi: 10.1016/j.apgeochem.2009.01.001 [47] 赵仁保, 孙海涛, 吴亚生, 等. 二氧化碳埋存对地层岩石影响的室内研究[J]. 中国科学: 技术科学, 2010, 40(4): 378-384. https://www.cnki.com.cn/Article/CJFDTOTAL-JEXK201004005.htmZhao R B, Sun H T, Wu Y S, et al. Influence of CO2 corrosion on rock structure and its mechanical characteristics[J]. Scientia Sinica(Technologica), 2010, 40(4): 378-384(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-JEXK201004005.htm [48] Ueda A, Ajima S, Yamamoto M. Isotopic study of carbonate minerals from the sumikawa geothermal area and its application to water movement[J]. Journal of the Geothermal Research Society of Japan, 2001, 23(3): 181-196. [49] 朱焕来, 曲希玉, 刘立, 等. CO2流体-长石相互作用实验研究[J]. 吉林大学学报: 地球科学版, 2011, 41(3): 697-706. https://www.cnki.com.cn/Article/CJFDTOTAL-CCDZ201103011.htmZhu H L, Qu X Y, Liu L, et al. Study on interaction between the feldspar and CO2 fluid[J]. Journal of Jilin University (Earth Science Edition), 2011, 41(3): 697-706(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-CCDZ201103011.htm [50] Xu T, Feng G, Shi Y. On fluid-rock chemical interaction in CO2-based geothermal systems[J]. Journal of Geochemical Exploration, 2014, 144: 179-193. doi: 10.1016/j.gexplo.2014.02.002 [51] 翔敖, 卢义玉, 汤积仁, 等. 页岩吸附CO2变形特性试验研究[J]. 煤炭学报, 2015, 40(12): 2893-2899.Xiang A, Lu Y Y, Tang J R, et al. Deformation properties of shale by sorbing carbon dioxide[J]. Journal of China Coal Society, 2015.40(12): 2893-2899(in Chinese with English abstract). [52] Jiang Y, Luo Y, Lu Y, et al. Effects of supercritical CO2 treatment time, pressure, and temperature on microstructure of shale[J]. Energy, 2016, 97: 173-181. doi: 10.1016/j.energy.2015.12.124 [53] 张臣, 周世新, 陈科, 等. 高压条件下CO2对页岩微观孔隙结构影响及其在页岩中的吸附特征[J]. 地球科学, 2019, 44(11): 3773-3782. https://www.cnki.com.cn/Article/CJFDTOTAL-DQKX201911017.htmZhang C, Zhou S X, Chen K, et al. Impact on microscopic pore structure and adsorption behavior of carbon dioxide on shale under high pressure Condition[J]. Earth Science, 2019, 44(11): 3773-3782(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-DQKX201911017.htm [54] 邹华耀, 吴时国. 有机质热成熟度指数理论与应用研究的新进展[J]. 天然气地球科学, 1992, 3(6): 1-8. https://www.cnki.com.cn/Article/CJFDTOTAL-TDKX199206000.htmZou H Y, Wu S G. New progress in theory and application of thermal maturity index of organic matter[J]. Natural Gas Geoscience, 1992, 3(6): 1-8(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-TDKX199206000.htm [55] Okamoto I, Li X, Ohsumi T. Effect of supercritical CO2 as the organic solvent on cap rock sealing performance for underground storage[J]. Energy, 2005, 30(11/12): 2344-2351. [56] Yin H, Zhou J, Jiang Y, et al. Physical and structural changes in shale associated with supercritical CO2 exposure[J]. Fuel, 2016, 184: 289-303. doi: 10.1016/j.fuel.2016.07.028 [57] Lyu Q, Long X, Pg R, et al. A laboratory study of geomechanical characteristics of black shales after sub-critical/super-critical CO2 brine saturation[J]. Geomechanics and Geophysics for Geo-Energy and Geo-Resources, 2018, 4: 141-156. doi: 10.1007/s40948-018-0079-5 [58] Zhang S, Xian X, Zhou J, et al. Mechanical behaviour of Longmaxi black shale saturated with different fluids: An experimental study[J]. Royal Society of Chemistry Advances. 2017, 7(68): 42946-42955. [59] Lee B, Rathnaweera T D. Stress threshold identification of progressive fracturing in Bukit Timah granite under uniaxial and triaxial stress conditions[J]. Geomechanics and Geophysics for Geo-Energy and Geo-Resources, 2016, 2(4): 301-330. doi: 10.1007/s40948-016-0037-z [60] Lu Y, Ao X, Tang J, et al. Swelling of shale in supercritical carbon dioxide[J]. Journal of Natural Gas Science & Engineering, 2016, 30: 268-275. [61] Xu T, Spycher N, Sonnenthal E, et al. TOUGHREACT Version 2.0: A simulator for subsurface reactive transport under non-isothermal multiphase flow conditions[J]. Computers & Geosciences, 2011, 37(6): 763-774. http://www.sciencedirect.com/science/article/pii/S0098300410003316 [62] Bächler D. Coupled thermal-hydraulic-chemical modelling at the Soultz-sous-Forêts HDR Reservoir (France)[D]. Zurich: Swiss Federal Institute of Technology Zurich, 2003. [63] Jörn Bartels, Cheng L Z, Clauser C, et al. Numerical simulation of reactive flow in hot aquifers[M]. Berlin Heidelberg: Springer, 2003. [64] Wolery T J. EQ3NR, a computer program for geochemical aqueous speciation-solubility calculations: theoretical manual, user's guide and related documentation (version 7.0). Part 3[M]. California: Lawrence Livermore Laboratory, University of California, 1992. [65] Xu T, Pruess K. Reactive transport modeling to study fluid-rock interactions in enhanced geothermal systems (EGS) with CO2 as working fluid[C]. Bali Indonesia: Proceedings World Geothermal Congress, 2010. [66] 那金, 许天福, 魏铭聪, 等. 增强地热系统热储层-盐水-CO2相互作用[J]. 吉林大学学报: 地球科学版, 2015, 45(5): 1493-1501. https://www.cnki.com.cn/Article/CJFDTOTAL-CCDZ201505021.htmNa J, Xu T F, Wei M C, et al. Interaction of rock-brine-supercritical CO2 in CO2-EGS reservoir[J]. Journal of Jilin University (Earth Science Edition), 2015, 45(5): 1493-1501(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-CCDZ201505021.htm [67] Nitschke F, Held S, Himmelsbach T, et al. THC simulation of halite scaling in deep geothermal single well production[J]. Geothermics, 2017, 65: 234-243. doi: 10.1016/j.geothermics.2016.09.009 [68] 陈继良, 黄文博, 曹文炅, 等. 增强型地热系统中液-岩化学作用数值模拟研究[J]. 新能源进展, 2016, 4(1): 48-55. doi: 10.3969/j.issn.2095-560X.2016.01.008Chen J L, Huang W B, Cao W J, et al. A numerical study on the effect of fluid-rock reaction during enhanced geothermal system heat extraction processes[J]. Advances in New and Renewable Energy, 2016, 4(1): 48-55(in Chinese with English abstract). doi: 10.3969/j.issn.2095-560X.2016.01.008 [69] Bethke C M, Yeakel S. The geochemist's workbench release 8.0: Reaction modeling Guide[M]. Champaign, Illinois: University of Illinois, 2009. [70] Reed M, Spycher N. Calculation of pH and mineral equilibria in hydrothermal waters with application to geothermometry and studies of boiling and dilution[J]. Geochimica et Cosmochimica Acta, 1984, 48(7): 1479-1492. doi: 10.1016/0016-7037(84)90404-6 -





 下载:
下载: