EEMs characteristics of dissolved organic matter in water environment and its implications for antimony contamination in antimony mine of Xikuangshan, Hunan Province
-
摘要:
溶解性有机质(dissolved organic matter, 简称DOM)是影响锑迁移转化的重要因素之一。湖南锡矿山锑矿是世界最大的锑矿, 水环境中锑污染情况严重。为查明锡矿山矿区水环境中DOM特征及其影响, 对锡矿山水环境样品进行三维荧光分析, 利用平行因子法提取水环境中天然有机组分, 分析荧光特征, 探究各组分之间与锑的相关关系。分析表明, 矿区水体环境中DOM以低腐殖化、陆源与微生物源混合来源为特点, 多数水样以陆源有机物为主。锡矿山锑矿区水体中包括了3种不同的组分: C1组分为陆源类腐殖质, C2组分为醌类腐殖质, C3组分为类蛋白质(酪氨酸); 水环境中C1组分相对含量最高, 地表水中C3组分相对含量高于地下水。研究认为, 存在以下途径影响地下水环境中锑的释放: ①类蛋白质组分与锑的络合促进锑的溶解释放; ②腐殖质组分与锑的直接络合。低锑地表水中天然有机组分相对含量受稀释作用的影响, 高锑地表水中天然有机组分相对含量对锑的来源有一定的指示作用。
Abstract:Dissolved organic matter (DOM) is an important factor affecting the migration and transformation of antimony(Sb). The Hunan Xikuangshan Sb mine is the largest Sb mine in the world and antimony pollution in the aquatic environment is serious. To determine the characteristics of DOM in the water environment of the Xikuangshan mining area and its influences, three-dimensional fluorescence analysis was performed on the water environment samples of the Xikuangshan mining area. PARAFAC was used to extract the natural organic components in the water environment and the relationship between each component and Sb was explored. The analysis shows that the DOM in the water environment of the mining area is characterized by low humification and mixed sources of terrestrial and microbial sources, and most water samples are mainly terrestrial organic matter. There are three different components in the water body of the Sb mining area of Xikuangshan: the C1 component is terrestrial humus; the C2 component is quinone humus; the C3 component is protein-like (tyrosine). The relative content of the C1 component in the water environment is the highest and the relative content of the C3 component in surface water is higher than that in groundwater. Studies have shown that there are the following ways to affect the release of Sb in the groundwater environment: (1) the complexation of protein-like components with Sb promotes the dissolution and release of Sb; (2) the direct complexation of humus components with Sb. The relative content of natural organic components in surface water with low Sb is affected by dilution. The relative content of natural organic components in surface water with high Sb has a certain indication for the source of Sb.
-
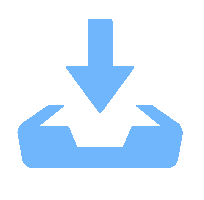
表 1 研究区地下水、地表水指标统计
Table 1. Statistics of indexes of groundwater and surface water in the study area
指标 锡矿山组 佘田桥组 地表水 最大值 最小值 平均值 最大值 最小值 平均值 最大值 最小值 平均值 pH 7.44 6.16 7.06 7.03 6.42 6.82 9.19 4.31 7.85 EC/(μS·cm-1) 1 237.0 231.0 593.0 1 119.0 189.4 570.0 1 018.0 297.0 588.7 Sb 2.70 0.015 0.49 7.45 0.51 2.51 5.39 0.028 1.60 HCO3- 492.3 45.4 251.1 257.5 36.4 153.3 181.8 104.5 146.0 SO42- 228.90 26.36 77.48 381.51 65.83 138.90 997.60 34.57 246.66 Cl- 27.42 4.41 8.09 8.85 3.10 5.27 293.66 3.60 25.67 Na+ 54.60 1.16 14.38 67.97 0.97 16.60 310.35 1.78 44.73 Ca2+ ρB/(mg·L-1) 128.72 27.64 78.51 124.85 22.61 72.86 160.16 36.66 73.15 K+ 9.72 0.78 3.62 5.57 1.22 3.73 9.40 1.63 4.25 Mg2+ 17.57 3.04 7.21 14.80 2.92 7.92 26.67 2.53 9.03 DOC 2.276 0.565 1.120 1.663 0.567 1.134 2.294 0.880 1.374 HIX 6.44 0.58 3.06 7.91 1.75 5.08 6.09 0.64 2.48 BIX 1.16 0.71 0.87 0.97 0.74 0.85 0.98 0.70 0.78 FI 1.90 1.52 1.65 1.75 1.57 1.66 1.67 1.51 1.57 SUVA254 2.00 -0.49 0.54 1.66 0 0.77 3.28 1.26 2.13 注: HIX.腐殖化指数; BIX.生物指数; FI.荧光指数; SUVA254.254 nm处UV的吸光系数与DOC浓度的比值 表 2 锡矿山水体中3个荧光组分特征及与前人研究确定组分的对比
Table 2. Characteristics of three components in the water environment of study area and their comparison with previously identified components
组分 (Ex/Em)/nm 本研究中DOM组分 前人研究中DOM组分描述 C1 235(295)/414 陆源类腐殖质 C2: < 240/416,类腐殖酸[7];
C1: < 236~260/400~500,陆源腐殖质[32];
C2:225(330)/410,陆地来源[36]C2 260/464 腐殖质类,醌类 C2: < 240~275/434~520,腐殖质类[32];
C3: < 250,366/468,醌类[25];
SQ2:270(375)/462,还原醌类[33]C3 220(270)/306 类蛋白质(酪氨酸) 220(270)/(300~305),类酪氨酸[37];
B峰:(225~237)/(309~321)和275/310,类酪氨酸[35];
C4:275/313,类酪氨酸[38]注:括号中数据为次级峰波长 -
[1] Wen B, Zhou A G, Zhou J W, et al. Coupled S and Sr isotope evidences for elevated arsenic concentrations in groundwater from the world's largest antimony mine, Central China[J]. Journal of Hydrology, 2018, 557(2): 211-221. [2] 江南, 李小倩, 周爱国, 等. pH值和氧化剂对硫化锑氧化溶解的影响机制[J]. 地质科技通报, 2020, 39(4): 76-84. doi: 10.19509/j.cnki.dzkq.2020.0410Jiang N, Li X Q, Zhou A G, et al. Effect of pH value and Fe(Ⅲ) on the oxidative dissolution of stibnite[J]. Bulletin of Geological Science and Technology, 2020, 39(4): 76-84 (in Chinese with English abstract). doi: 10.19509/j.cnki.dzkq.2020.0410 [3] 张玲, 徐胜, 邱国院, 等. 巍山某锑矿厂周围水土中锑含量的检测分析[J]. 大理学院学报, 2008, 7(12): 37-38, 42.Zhang L, Xu S, Qiu G Y, et al. Detection and analysis of the content of antimony in the water and soil around an antimony ore factory in Weishan[J]. Journal of Dali University, 2008, 7(12): 37-38, 42 (in Chinese with English abstract). [4] 朱静, 吴丰昌, 邓秋静, 等. 湖南锡矿山周边水体的环境特征[J]. 环境科学学报, 2009, 29(3): 655-661. doi: 10.3321/j.issn:0253-2468.2009.03.029Zhu J, Wu F C, Deng Q J, et al. Environmental characteristics of water near the Xikuangshan antimony mine, Hunan Province[J]. Acta Scientiae Circumstantiae, 2009, 29(3): 655-661 (in Chinese with English abstract). doi: 10.3321/j.issn:0253-2468.2009.03.029 [5] 宁增平, 肖唐付, 杨菲, 等. 锑矿区水体水环境锑污染及硫同位素示踪研究[J]. 矿物岩石地球化学通报, 2011, 30(2): 135-141. doi: 10.3969/j.issn.1007-2802.2011.02.003Ning Z P, Xiao T F, Yang F, et al. Antimony pollution and sulfur isotope study in the waters of an antimony mine area[J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2011, 30(2): 135-141 (in Chinese with English abstract). doi: 10.3969/j.issn.1007-2802.2011.02.003 [6] Wang X Q, He M C, Xi J H, et al. Antimony distribution and mobility in rivers around the world's largest antimony mine of Xikuangshan, Hunan Province, China[J]. Microchemical Journal, 2010, 97(1): 4-11. [7] Stedmon C A, Markager S, Bro R. Tracing dissolved organic matter in aquatic environments using a new approach the fluorescence spectroscopy[J]. Marine Chemistry, 2003, 82(3): 239-254. [8] Murphy K R, Butler K D, Spencer R G M, et al. Measurement of dissolved organic matter fluorescence in aquatic environments: An interlaboratory comparison[J]. Environmental Science & Technology, 2010, 44(24): 9405-9412. [9] Tella M, Pokrovski G S. Antimony(Ⅲ) complexing with o-bearing organic ligands in aqueous solution: An X-ray absorption fine structure spectroscopy and solubility study[J]. Geochimica et Cosmochimica Acta, 2008, 73(2): 268-290. [10] Pilarski J, Waller P, Pickering W. Sorption of antimony species by humic acid[J]. Water, Air & Soil Pollution, 1995, 84(1/2): 51-59. [11] Tighe M, Lockwood P, Wilson S. Adsorption of antimony(V) by floodplain soils, amorphous iron(III) hydroxide and humic acid[J]. Journal of Environmental Monitoring: JEM, 2005, 7(12): 1177-1185. doi: 10.1039/b508302h [12] Buschmann J, Canonica S, Sigg L. Photoinduced oxidation of antimony(III) in the presence of humic acid[J]. Environmental Science & Technology, 2005, 39(14): 5335-5341. [13] Wu T L, Qin W X, Alves M E, et al. Mechanisms of Sb(III) oxidation mediated by low molecular weight phenolic acids[J]. Chemical Engineering Journal, 2019, 356: 190-198. doi: 10.1016/j.cej.2018.09.008 [14] Buschmann J, Sigg L. Antimony(III) binding to humic substances: Influence of pH and type of humic acid[J]. Environmental Science & Technology, 2004, 38(17): 4535-4541. [15] Hu X Y, He M C. Organic ligand-induced dissolution kinetics of antimony trioxide[J]. Journal of Environmental Sciences, 2017, 56: 87-94. doi: 10.1016/j.jes.2016.09.006 [16] Karimian N, Edward D B, Scott G J. Antimony speciation and mobility during Fe(II)-induced transformation of humic acid-antimony(V)-iron(III) coprecipitates[J]. Environmental Pollution, 2019, 254(113): 112-121. [17] Migon C, Mori C. Arsenic and antimony release from sediments in a Mediterranean Estuary[J]. Hydrobiologia, 1999, 392(1): 81-88. doi: 10.1023/A:1003561609548 [18] Deng T L, Chen Y W, Belzile N. Antimony speciation at ultra trace levels using hydride generation atomic fluorescence spectrometry and 8-hydroxyquinoline as an efficient masking agent[J]. Analytica Chimica Acta, 2001, 432(2): 293-302. doi: 10.1016/S0003-2670(00)01387-8 [19] Heier L S, Meland S, Ljønes M, et al. Short-term temporal variations in speciation of Pb, Cu, Zn and Sb in a shooting range runoff stream[J]. Science of the Total Environment, 2010, 408(11): 2409-2417. doi: 10.1016/j.scitotenv.2010.02.019 [20] 陈诗雨, 李燕, 李爱民. 溶解性有机物研究中三维荧光光谱分析的应用[J]. 环境科学与技术, 2015, 38(5): 64-68, 73. https://www.cnki.com.cn/Article/CJFDTOTAL-FJKS201505014.htmChen S Y, Li Y, Li A M. Application of three-dimensional fluorescence spectroscopy in the study of dissolved organic matter[J]. Environmental Science & Technology, 2015, 38(5): 64-68, 73 (in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-FJKS201505014.htm [21] 田巍, 陈孝红, 李旭兵, 等. 湘中涟源凹陷下石炭统天鹅坪组页岩气成藏条件及主控因素[J]. 地质科技通报, 2021, 40(5): 54-63. doi: 10.19509/j.cnki.dzkq.2021.0504Tian W, Chen X H, Li X B, et al. Accumulation conditions and main factors of the Lower Carboniferous Tianeping Formation in Lianyuan Sag in the middle of Hunan Province[J]. Bulletin of Geological Science and Technology, 2021, 40(5): 54-63(in Chinese with English abstract). doi: 10.19509/j.cnki.dzkq.2021.0504 [22] 弭希风, 胡瑞忠, 付山岭, 等. 湖南锡矿山超大型锑矿床围岩蚀变元素迁移特征及定量计算研究[J]. 矿物岩石地球化学通报, 2019, 38(1): 103-113. https://www.cnki.com.cn/Article/CJFDTOTAL-KYDH201901011.htmMi X F, Hu R Z, Fu S L, et al. Elemental migration and mass balance calculation of wall rock alternation in the Xikuangshan Sb deposit, Hunan Province[J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2019, 38(1): 103-113 (in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-KYDH201901011.htm [23] 黄爽兵, 王焰新, 刘昌蓉, 等. 含水层中砷活化迁移的水化学与DOM三维荧光证据[J]. 地球科学: 中国地质大学学报, 2013, 38(5): 1091-1098. https://www.cnki.com.cn/Article/CJFDTOTAL-DQKX201305021.htmHuang S B, Wang Y X, Liu C R, et al. Hydrochemical and fluorescent spectroscopic evidences of arsenic mobilization in groundwater[J]. Earth Science: Journal of China University of Geosciences, 2013, 38(5): 1091-1098 (in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-DQKX201305021.htm [24] 鲁宗杰, 邓娅敏, 杜尧, 等. 江汉平原高砷地下水中DOM三维荧光特征及其指示意义[J]. 地球科学, 2017, 42(5): 771-782.Lu Z J, Deng Y M, Du Y, et al. EEMs characteristics of dissolved organic matter and their implication in high arsenic groundwater of Jianghan Plain[J]. Earth Science, 2017, 42(5): 771-782 (in Chinese with English abstract). [25] 梁梦钰, 郭华明, 李晓萌, 等. 贵德盆地三河流域高砷地下水中溶解性有机物三维荧光特性及其指示意义[J]. 地学前缘, 2019, 26(3): 243-254. https://www.cnki.com.cn/Article/CJFDTOTAL-DXQY201903034.htmLiang M Y, Guo H M, Li X M, et al. Excitation-emission matrix spectroscopic characteristics of dissolved organic matters and the significance in high arsenic groundwater research in the Guide Basin, China[J]. Earth Science Frontiers, 2019, 26(3): 243-254 (in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-DXQY201903034.htm [26] Huguet A, Vacher L, Relexans S, et al. Properties of fluorescent dissolved organic matter in the Gironde Estuary[J]. Organic Geochemistry, 2009, 40(6): 706-719. doi: 10.1016/j.orggeochem.2009.03.002 [27] 席玥, 王婷, 倪晋仁, 等. 黄河干流宁蒙段溶解性有机物组分特征及其与金属离子的相关性[J]. 环境科学, 2018, 39(9): 4114-4121. https://www.cnki.com.cn/Article/CJFDTOTAL-HJKZ201809021.htmXi Y, Wang T, Ni J R, et al. Characterization of dissolved organic matter fractions in the Ning-Meng section of Yellow River and relationship with metal ions[J]. Environmental Science, 2018, 39(9): 4114-4121 (in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-HJKZ201809021.htm [28] McKnight D M, Boyer E W, Westerhoff P K, et al. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity[J]. Limnology and Oceanography, 2001, 46(1): 38-48. doi: 10.4319/lo.2001.46.1.0038 [29] Weishaar J L, Aiken G R, Bergamaschi B A, et al. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon[J]. Environmental Science & Technology, 2003, 37(20): 4702-4708. [30] Edzwald J K, Tobiason J E. Enhanced coagulation: US requirements and a broader view[J]. Water Science and Technology, 1999, 40(9): 63-70. doi: 10.2166/wst.1999.0444 [31] Dilling J, Kaiser K. Estimation of the hydrophobic fraction of dissolved organic matter in water samples using UV photometry[J]. Water Research, 2002, 36(20): 5037-5044. doi: 10.1016/S0043-1354(02)00365-2 [32] Ishii S K L, Boyer T H. Behavior of reoccurring PARAFAC components in fluorescent dissolved organic matter in natural and engineered systems: A critical review[J]. Environmental Science & Technology, 2012, 46(4): 2006-2017. [33] Cory R M, McKnight D M. Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter[J]. Environmental Science & Technology, 2005, 39(21): 8142-8149. [34] Singh S, Inamdar S, Scott D, et al. Comparison of two PARAFAC models of dissolved organic matter fluorescence for a Mid-Atlantic Forested Watershed in the USA[J]. Journal of Ecosystems, 2013, 2013: 532424. [35] Hudson N, Baker A, Reynolds D. Fluorescence analysis of dissolved organic matter in natural, waste and polluted waters-A review[J]. River Research and Applications, 2007, 23(6): 631-649. doi: 10.1002/rra.1005 [36] Carstea E M, Baker A, Bieroza M, et al. Characterisation of dissolved organic matter fluorescence properties by PARAFAC analysis and thermal quenching[J]. Water Research, 2014, 61: 152-161. doi: 10.1016/j.watres.2014.05.013 [37] Coble P G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy[J]. Marine Chemistry, 1996, 51(4): 325-346. doi: 10.1016/0304-4203(95)00062-3 [38] Yamashita Y, Kloeppel B D, Knoepp J, et al. Effects of watershed history on dissolved organic matter characteristics in headwater streams[J]. Ecosystems, 2011, 14(7): 1110-1122. doi: 10.1007/s10021-011-9469-z [39] Wang Y, Zhang D, Shen Z Y, et al. Investigation of the interaction between As and Sb species and dissolved organic matter in the Yangtze Estuary, China, using excitation-emission matrices with parallel factor analysis[J]. Environmental Science and Pollution Research, 2015, 22(3): 1819-1830. doi: 10.1007/s11356-014-3380-z [40] Li X D, Wu B, Zhang Q, et al. Complexation of humic acid with Fe ions upon persulfate / ferrous oxidation: Further insight from spectral analysis[J]. Journal of Hazardous Materials, 2020, 399(123): 71-78. -





 下载:
下载: