Effects of seasonal variation in organic matter in groundwater on reactive nitrogen transport in the Jianghan Plain
-
摘要:
溶解性有机质(DOM)是地下水中生物地球化学过程的重要碳源。为阐明江汉平原地下水中DOM季节性变化对N迁移转化的影响, 选取江汉平原沙湖监测场作为研究区, 根据地下水、地表水长期水位和水化学监测数据, 进行水文地球化学分析, 联合三维荧光光谱和紫外可见光谱, 分析DOM季节性变化特征, 探究了水文条件影响下地下水中DOM季节变化在N迁移转化中的作用。研究结果表明: 研究区地下水和地表水中DOM包括3种组分: 陆源类腐殖酸(C1)、微生物源类色氨酸(C2)和微生物源类腐殖酸(C3)。枯水期微生物源类色氨酸组分输入增加, 丰水期陆源类腐殖酸组分输入增加。研究区地下水的强还原性和高溶解性有机碳(DOC)含量为硝酸盐的还原提供了条件, 低腐殖化、低分子量的C2组分在N迁移转化中优先被利用。枯水期, 地下水水位下降, 含水层偏氧化性, 不稳定的类蛋白组分快速降解释放NH4-N, 硝化反应、有机氮矿化速率较高, 反硝化、硝酸盐异化还原为氨(DNRA)反应速率较低; 丰水期, 地下水水位上升, 含水层偏还原性, 硝化作用受到抑制, 大量不易被降解的DOM存在使含水层中有机氮矿化速率降低, 反硝化和DNRA过程被促进。综上所述, 研究区DOM季节性变化是控制地下水中N反应迁移的重要因素。
Abstract:Objective Dissolved organic matter (DOM) is an important carbon source in the biogeochemical process of groundwater.
Methods To reveal the impact of the seasonal variation in DOM on the migration and transformation in groundwater on N in the Jianghan Plain, long-term water level and hydrochemical data of groundwater and surface water at the Shahu monitoring site were obtained, and the hydrogeochemistry analysis was carried out. The seasonal variation characteristics of DOM were analysed by combing with three-dimensional fluorescence spectroscopy and UV-V is spectroscopy, to explore the role of DOM in groundwater in N migration and transformation under the influence of hydrological conditions.
Results The results show that DOM in groundwater and surface water includes three components: terrestrial humic-like component (C1), microbial tryptophan-like component (C2) and microbial humic-like component (C3). The input of microbial tryptophan-like components increases in dry season and terrestrial humic-like components increase in wet season. The strong reducibility and high dissolved organic carbon(DOC) content of groundwater provide conditions for the nitrate reduction, and low humification and low molecular weight C2 components are preferentially utilized in N migration and transformation. In dry season, the groundwater level decreases, the aquifer is partial to oxidation, the unstable protein-like components quickly degrade and release NH4-N, the nitrification and organic nitrogen mineralization rates are higher, and the denitrification and dissimilatory nitrite reduction to ammonium(DNRA) reaction rates are lower. In the wet season, the groundwater level rises, the aquifer tends to be reductive, and nitrification is inhibited. The presence of a large amount of DOM that is not easy to be degraded reduces the mineralization reaction rate of organic nitrogen in the aquifer, and the denitrification and DNRA processes are promoted.
Conclusion In summary, the seasonal variation in DOM in the study area is an important factor in controlling the migration and transformation of N in groundwater.
-
Key words:
- Jianghan Plain /
- groundwater /
- dissolved organic matter /
- seasonal variation /
- nitrogen
-
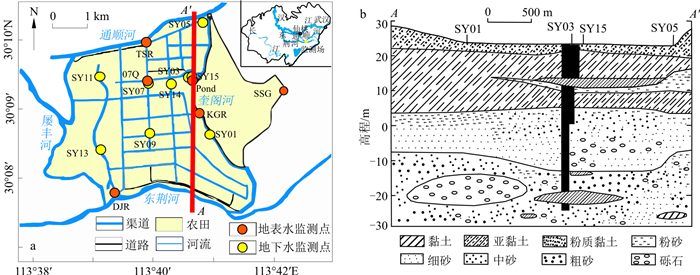
图 1 研究区概况和采样点位置(a)和水文地质剖面图(b) (根据文献[26]修改)
Figure 1. Overview of the study area and location of the sampling sites (a), and hydrogeological profile (b)
表 1 研究区地下水主要水化学指标统计
Table 1. Statistics of groundwater chemistry in the study area
指标 井深/m 枯水期 丰水期 最小值~最大值(平均值) 最小值~最大值(平均值) pH 10 7.06~7.82(7.42) 6.41~7.63(7.01) 25 7.22~8.07(7.60) 6.52~7.90(7.17) 50 6.98~7.88(7.53) 6.37~7.76(7.16) EC/(μS·cm-1) 10 215.40~1 178.00(863.44) 204.70~1 568.00(1 041.57) 25 141.10~1 002.00(694.91) 150.90~1 366.00(835.55) 50 175.50~883.00(616.97) 194.70~1 157.00(750.39) ORP/mV 10 -81.30~188.00(-18.50) -169.70~131.80(-60.49) 25 -127.90~188.80(-5.94) -163.80~104.30(-53.74) 50 -170.70~185.00(-15.18) -166.20~104.50(-60.21) DO 10 0.72~3.43(1.79) 0.05~2.93(0.84) 25 0.69~5.48(2.24) 0.08~2.24(0.98) 50 0.39~5.96(1.86) 0.22~1.97(0.83) DOC 10 2.42~11.09(5.48) 2.11~9.81(5.42) 25 1.87~18.64(4.43) 2.37~23.73(4.56) 50 1.85~7.33(3.50) 1.52~6.15(3.47) DIC ρB/(mg·L-1) 10 26.58~145.88(68.03) 30.22~258.53(132.33) 25 18.17~106.62(60.87) 43.03~173.68(104.66) 50 15.86~92.37(52.85) 23.18~183.03(90.22) Cl- 10 6.75~63.26(21.96) 6.08~72.66(23.28) 25 4.49~30.43(8.65) 2.85~29.50(8.11) 50 4.23~93.11(15.76) 2.95~57.86(15.94) SO42- 10 0.00~41.05(7.40) 0.00~41.96(7.02) 25 0.11~56.29(9.34) 0.00~49.70(10.56) 50 0.00~38.58(7.44) 0.00~36.85(8.90) S2- ρB/(μg·L-1) 10 1.00~54.00(16.59) 0.00~841.00(62.81) 25 0.00~637.00(58.48) 0.00~67.00(14.63) 50 0.00~772.00(75.44) 0.00~268.00(30.70) K+ 10 1.41~22.68(4.84) 0.85~18.14(3.23) 25 1.36~63.37(8.24) 0.53~62.74(8.34) 50 0.90~35.75(6.75) 0.82~35.28(5.95) Na+ 10 2.33~37.03(20.33) 2.24~39.07(21.59) 25 1.42~35.47(18.96) 1.40~35.10(19.22) 50 2.25~48.44(18.94) 2.27~54.33(19.52) Ca2+ 10 45.02~213.74(144.50) 38.91~251.47(158.37) 25 31.39~187.15(124.58) 30.08~205.37(125.26) 50 37.16~229.49(108.62) 37.12~208.88(109.97) Mg2+ 10 4.79~77.93(34.01) 4.01~79.32(35.83) 25 2.31~33.01(23.11) 2.23~34.87(23.10) 50 3.99~36.46(21.28) 4.00~34.67(21.27) HCO3- ρB/(mg·L-1) 10 167~1037(639) 119~1051(691) 25 88~770(529) 98~742(539) 50 123~782(463) 119~819(472) Fe 10 0.83~26.20(6.38) 0.00~15.40(5.89) 25 0.07~12.20(4.09) 0.05~9.66(3.96) 50 0.35~7.65(4.12) 0.41~8.30(3.48) Fe2+ 10 0.04~14.90(4.30) 0.00~9.55(4.09) 25 0.00~8.95(3.02) 0.00~7.45(1.90) 50 0.01~6.60(2.96) 0.00~5.05(1.82) NO3-N 10 0.00~1.83(0.36) 0.00~2.15(0.17) 25 0.00~1.24(0.26) 0.00~3.46(0.68) 50 0.00~0.82(0.12) 0.00~3.03(0.84) NO2-N 10 0.00~0.10(0.02) 0.00~0.09(0.01) 25 0.00~0.06(0.01) 0.00~0.05(0.02) 50 0.00~0.10(0.02) 0.00~0.04(0.02) NH4-N ρB/(mg·L-1) 10 0.19~9.35(3.39) 0.18~7.85(3.52) 25 0.05~4.96(2.27) 0.04~4.78(2.14) 50 0.04~5.45(2.49) 0.27~5.40(1.86) δ18O/‰ 10 -8.87~-5.65(-7.31) -12.35~-5.77(-7.49) 25 -11.22~-5.20(-7.78) -9.53~-5.13(-7.26) 50 -11.92~-4.46(-7.90) -11.56~-4.76(-7.52) δ2H/‰ 10 -53.11~-37.44(-44.61) -86.22~-32.98(-47.42) 25 -74.07~-23.33(-46.81) -64.88~-25.46(-44.58) 50 -79.77~-33.12(-49.06) -80.24~-24.29(-47.21) -
[1] Fellman J B, Hood E D, Amore D V, et al. Seasonal changes in the chemical quality and biodegradability of dissolved organic matter exported from soils to streams in coastal temperate rainforest watersheds[J]. Biogeochemistry, 2009, 95(2/3): 277-293. [2] Yamashita Y, Jaffé R, Maie N, et al. Assessing the dynamics of dissolved organic matter (DOM) in coastal environments by excitation emission matrix fluorescence and parallel factor analysis (EEM-PARAFAC)[J]. Limnology & Oceanography, 2008, 53(5): 1900-1908. [3] Catalán N, Obrador B, Alomar C, et al. Seasonality and landscape factors drive dissolved organic matter properties in Mediterranean ephemeral washes[J]. Biogeochemistry, 2013, 112(1/3): 261-274. [4] Larsen L G, Aiken G R, Harvey J W, et al. Using fluorescence spectroscopy to trace seasonal DOM dynamics, disturbance effects, and hydrologic transport in the Florida Everglades[J]. Journal of Geophysical Research Atmospheres, 2010, 115: G03001. [5] 何小松, 席北斗, 张鹏, 等. 地下水中溶解性有机物的季节变化特征及成因[J]. 中国环境科学, 2015, 35(3): 862-870. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGHJ201503039.htmHe X S, Xi B D, Zhang P, et al. The seasonal distribution characteristics and its reasons of dissolved organic matter in groundwater[J]. Chinese Environmental Science, 2015, 35(3): 862-870 (in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-ZGHJ201503039.htm [6] Raymond P A, Mcclelland J W, Holmes R M, et al. Flux and age of dissolved organic carbon exported to the Arctic Ocean: A carbon isotopic study of the five largest arctic rivers[J]. Global Biogeochemical Cycles, 2007, 21(4): GB4011. [7] Cai Y H, Guo L D, Wang X R, et al. Abundance, stable isotopic composition, and export fluxes of DOC, POC, and DIC from the Lower Mississippi River during 2006-2008[J]. Journal of Geophysical Research Biogeosciences, 2016, 120(11): 2273-2288. [8] Smith M A, Kominoski J S, Gaiser E E, et al. Stormwater runoff and tidal flooding transform dissolved organic matter composition and increase bioavailability in urban coastal ecosystems[J]. Journal of Geophysical Research Biogeosciences, 2021, 126(7): e2020JG006146. [9] Cárdenas C S, Gerea M, Garcia P E, et al. Interplay between climate and hydrogeomorphic features and their effect on the seasonal variation of dissolved organic matter in shallow temperate lakes of the Southern Andes (Patagonia, Argentina): A field study based on optical properties[J]. Ecohydrology, 2017, 10(7): e1872. doi: 10.1002/eco.1872 [10] Yang Y J, Yuan X F, Deng Y M, et al. Seasonal dynamics of dissolved organic matter in high arsenic shallow groundwater systems[J]. Journal of Hydrology, 2020, 589: 125120. doi: 10.1016/j.jhydrol.2020.125120 [11] Chen M, Maie N, Parish K, et al. Spatial and temporal variability of dissolved organic matter quantity and composition in an oligotrophic subtropical coastal wetland[J]. Biogeochemistry, 2013, 115(1/3): 167-183. [12] Mladenov N, Philippa H, Wolski P, et al. Dissolved organic matter accumulation, reactivity, and redox state in ground water of a recharge wetland[J]. Wetlands, 2008, 28(3): 747-759. doi: 10.1672/07-140.1 [13] Xiong Y J, Du Y, Deng Y M, et al. Contrasting sources and fate of nitrogen compounds in different groundwater systems in the Central Yangtze River Basin[J]. Environmental Pollution, 2021, 290: 118119. doi: 10.1016/j.envpol.2021.118119 [14] Donn M J, Barron O V. Biogeochemical processes in the groundwater discharge zone of urban streams[J]. Biogeochemistry, 2013, 115(1/3): 267-286. [15] 张董涛, 刘璐, 马腾, 等. 黏性土弱透水层氮形态的赋存特征及迁移转化: 以江汉平原沉湖沉积物为例[J]. 安全与环境工程, 2020, 27(3): 118-125. https://www.cnki.com.cn/Article/CJFDTOTAL-KTAQ202003017.htmZhang D T, Liu L, Ma T, et al. Occurrence, migration and transformation characteristics of nitrogen forms in clay aquitards[J]. Safety and Environmental Engineering, 2020, 27(3): 118-125(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-KTAQ202003017.htm [16] Tye A M, Lapworth D J. Characterising changes in fluorescence properties of dissolved organic matter and links to N cycling in agricultural floodplains[J]. Agriculture, Ecosystems & Environment, 2016, 221: 245-257. [17] Bonin P. Anaerobic nitrate reduction to ammonium in two strains isolated from coastal marine sediment: A dissimilatory pathway[J]. Fems Microbiology Ecology, 1996, 19(1): 27-38. doi: 10.1111/j.1574-6941.1996.tb00195.x [18] Shvartsev S L, Liu H, Kamaletdinova L L. Ecological and geochemical conditions of the groundwater in the Jianghan Basin, Hubei Province, China[J]. Aqua Mundi, 2012, 47(6): 135-142. [19] Huang S B, Wang Y X, Cao L, et al. Multidimensional spectrofluorometry characterization of dissolved organic matter in arsenic-contaminated shallow groundwater[J]. Journal of Environmental Science and Health Part A Toxic/Hazardous Substances & Environmental Engineering, 2012, 47(10): 1446-1454. [20] Du Y, Ma T, Deng Y M, et al. Sources and fate of high levels of ammonium in surface water and shallow groundwater of the Jianghan Plain, Central China[J]. Environmental Science: Processes & Impacts, 2017, 19(2): 161-172. doi: 10.3969/j.issn.1673-1212.2017.02.038 [21] Liang Y, Ma R, Wang Y X, et al. Hydrogeological controls on ammonium enrichment in shallow groundwater in the central Yangtze River Basin[J]. Science of the Total Environment, 2020, 741: 140350. doi: 10.1016/j.scitotenv.2020.140350 [22] Sun L Q, Liang X, Jin M G, et al. Ammonium and nitrate sources and transformation mechanism in the Quaternary sediments of Jianghan Plain, China[J]. Science of the Total Environment, 2021, 774: 145131. doi: 10.1016/j.scitotenv.2021.145131 [23] 沈帅, 马腾, 杜尧, 等. 江汉平原典型地区季节性水文条件影响下氮的动态变化规律[J]. 地球科学, 2017, 42(5): 674-684. https://www.cnki.com.cn/Article/CJFDTOTAL-DQKX201705002.htmShen S, Ma T, Du Y, et al. Dynamic variations of nitrogen in groundwater under influence of seasonal hydrological condition in typical area of Jianghan Plain[J]. Earth Science, 2017, 42(5): 674-684(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-DQKX201705002.htm [24] 梁杏, 张婧玮, 蓝坤, 等. 江汉平原地下水化学特征及水流系统分析[J]. 地质科技通报, 2020, 39(1): 21-33. doi: 10.19509/j.cnki.dzkq.2020.0103Liang X, Zhang J W, Lan K, et al. Hydrochemical characteristics of groundwater and analysis of groundwater flow systems in Jianghan Plain[J]. Bulletin of Geological Science and Technology, 2020, 39(1): 21-33(in Chinese with English abstract). doi: 10.19509/j.cnki.dzkq.2020.0103 [25] Gan Y Q, Zhao K, Deng Y M, et al. Groundwater flow and hydrogeochemical evolution in the Jianghan Plain, central China[J]. Hydrogeology Journal, 2018, 26(5): 1609-1623. doi: 10.1007/s10040-018-1778-2 [26] 李红梅, 邓娅敏, 罗莉威, 等. 江汉平原高砷含水层沉积物地球化学特征[J]. 地质科技情报, 2015, 34(3): 178-184. https://www.cnki.com.cn/Article/CJFDTOTAL-DZKQ201503025.htmLi H M, Deng Y M, Luo L W, et al. Geochemical characteristics of sediments from high arsenic aquifers in Jianghan Plain[J]. Geological Science and Technology Information, 2015, 34(3): 178-184(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-DZKQ201503025.htm [27] 王妍妍, 黄爽兵, 赵龙, 等. 江汉平原沙湖地区浅层含水层第四纪沉积环境演化[J]. 地球科学, 2017, 42(5): 751-760. https://www.cnki.com.cn/Article/CJFDTOTAL-DQKX201705010.htmWang Y Y, Huang S B, Zhao L, et al. Evolution of Quaternary sedimentary environment in shallow aquifers, at Shahu area, Jianghan Plain[J]. Earth Science, 2017, 42(5): 751-760(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-DQKX201705010.htm [28] 赵德君. 江汉平原地下水系统三维数值模拟[D]. 武汉: 中国地质大学(武汉), 2005.Zhao D J. The three-dimensional numerical simulation for groundwater system in Jianghan Plain[D]. Wuhan: China University of Geosciences(Wuhan), 2005(in Chinese with English abstract). [29] 甘义群, 王焰新, 段艳华, 等. 江汉平原高砷地下水监测场砷的动态变化特征分析[J]. 地学前缘, 2014, 21(4): 37-49. https://www.cnki.com.cn/Article/CJFDTOTAL-DXQY201404006.htmGan Y Q, Wang Y X, Duan Y H, et al. Dynamic changes of groundwater arsenic concentration in the monitoring field site, Jianghan Plain[J]. Earth Science Frontiers, 2014, 21(4): 37-49(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-DXQY201404006.htm [30] Stedmon C A, Markager S. Resolving the variability in dissolved organic matter fluorescence in a temperate estuary and its catchment using PARAFAC analysis[J]. Limnology & Oceanography, 2005, 50(2): 686-697. [31] Mcknight D M, Boyer E W, Westerhoff P K, et al. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity[J]. Limnology & Oceanography, 2001, 46(1): 38-48. [32] Huguet A, Vacher L, Relexans S, et al. Properties of fluorescent dissolved organic matter in the gironde estuary[J]. Organic Geochemistry, 2008, 40(6): 706-719. [33] Zsolnay A, Baigar E, Jimenez M, et al. Differentiating with fluorescence spectroscopy the sources of dissolved organic matter in soils subjected to drying[J]. Chemosphere, 1999, 38(1): 45-50. doi: 10.1016/S0045-6535(98)00166-0 [34] Zhang Y L, Liu M L, Qin B Q, et al. Photochemical degradation of chromophoric-dissolved organic matter exposed to simulated UV-B and natural solar radiation[J]. Hydrobiologia, 2009, 627(1): 159-168. doi: 10.1007/s10750-009-9722-z [35] Fichot C G, Benner R. The spectral slope coefficient of chromophoric dissolved organic matter (S275-295) as a tracer of terrigenous dissolved organic carbon in river-influenced ocean margins[J]. Limnology and Oceanography, 2012, 57(5): 1453-1466. doi: 10.4319/lo.2012.57.5.1453 [36] Hansen A M, Kraus T E C, Pellerin B A, et al. Optical properties of dissolved organic matter (DOM): Effects of biological and photolytic degradation[J]. Limnology and Oceanography, 2016, 61(3): 1015-1032. [37] Ma F, Wang G L, Sun H L, et al. Indication of hydrogen and oxygen stable isotopes on the characteristics and circulation patterns of medium-low temperature geothermal resources inthe Guanzhong Basin, China[J]. Journal of Groundwater Science and Engineering, 2022, 10(1): 70-86. [38] 赵家成, 魏宝华, 肖尚斌. 湖北宜昌地区大气降水中的稳定同位素特征[J]. 热带地理, 2009, 29(6): 526-531. https://www.cnki.com.cn/Article/CJFDTOTAL-RDDD200906006.htmZhao J C, Wei B H, Xiao S B, et al. Stable isotope characteristics of atmospheric precipitation in Yichang, Hubei Province[J]. Tropical Geography, 2009, 29(6): 526-531 (in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-RDDD200906006.htm [39] 江欣悦, 李静, 郭林, 等. 豫北平原浅层地下水化学特征与成因机制[J]. 地质科技通报, 2021, 40(5): 290-300. doi: 10.19509/j.cnki.dzkq.2021.0511Jiang X Y, Li J, Guo L, et al. Chemical characteristics and genetic mechanism of shallow groundwater in Northern Henan Plain[J]. Bulletin of Geological Science and Technology, 2021, 40(5): 290-300 (in Chinese with English abstract). doi: 10.19509/j.cnki.dzkq.2021.0511 [40] 段艳华, 甘义群, 郭欣欣, 等. 江汉平原高砷地下水监测场水化学特征及砷富集影响因素分析[J]. 地质科技情报, 2014, 33(2): 140-147. https://www.cnki.com.cn/Article/CJFDTOTAL-DZKQ201402024.htmDuan Y H, Gan Y Q, Guo X X, et al. Analysis of hydrochemical characteristics and influencing factors of arsenic enrichment in high arsenic groundwater monitoring field in Jianghan Plain[J]. Geological Science and Technology Information, 2014, 33(2): 140-147(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-DZKQ201402024.htm [41] 于凯. 高砷地下水系统中有机质来源及其对砷动态变化的影响研究[D]. 武汉: 中国地质大学(武汉), 2016.Yu K. The sources and influences of dissolved organic matter on temporal variations of groundwater arsenic concentrations: A case study in Jianghan Plain[D]. Wuhan: China University of Geosciences(Wuhan), 2016(in Chinese with English abstract). [42] Liu X W, Wang H Y, Zhou J M, et al. Effect of N fertilization pattern on rice yield, N use efficiency and fertilizer-N fate in the Yangtze River Basin, China[J]. Plos One, 2016, 11(11): e0166002. [43] Gao Z P, Weng H C, Guo H M. Unraveling influences of nitrogen cycling on arsenic enrichment in groundwater from the Hetao Basin using geochemical and multi-isotopic approaches[J]. Journal of Hydrology, 2021, 595: 125981. [44] Coble P G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy[J]. Marine Chemistry, 1996, 51(4): 325-346. [45] Fellman J B, Hood E, Spencer R. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review[J]. Limnology & Oceanography, 2010, 55(6): 2452-2462. [46] Huang S B, Wang Y X, Ma T, et al. Linking groundwater dissolved organic matter to sedimentary organic matter from a fluvio-lacustrine aquifer at Jianghan Plain, China by EEM-PARAFAC and hydrochemical analyses[J]. Science of the Total Environment, 2015, 529: 131-139. [47] Singh S, Dash P, Silwal S, et al. Influence of land use and land cover on the spatial variability of dissolved organic matter in multiple aquatic environments[J]. Environmental Science and Pollution Research, 2017, 24(16): 14124-14141. [48] Cory R M, Mcknight D M. Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter[J]. Environmental Science & Technology, 2005, 39(21): 8142-8149. [49] Stedmon C A, Markager S, Bro R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy[J]. Marine Chemistry, 2003, 82(3/4): 239-254. [50] Wu F C, Evans R D, Dillon P J. Separation and characterization of NOM by high-performance liquid chromatography and on-line three-dimensional excitation emission matrix fluorescence detection[J]. Environmental Science & Technology, 2003, 37(16): 3687-3693. [51] Fellman J B, Hood E, Edwards R T, et al. Changes in the concentration, biodegradability, and fluorescent properties of dissolved organic matter during stormflows in coastal temperate watersheds[J]. Journal of Geophysical Research, 2009, 114: G01021. [52] Miadenov N, Zheng Y, Simone B, et al. Dissolved organic matter quality in a shallow aquifer of Bangladesh: Implications for arsenic mobility[J]. Environmental Science & Technology, 2015, 49(18): 10815-10824. [53] Hu H D, Xing X Y, Wang J F, et al. Characterization of dissolved organic matter in reclaimed wastewater supplying urban rivers with a special focus on dissolved organic nitrogen: A seasonal study[J]. Environmental Pollution, 2020, 265: 114959. [54] Lafrenière M, Lamoureux S. Seasonal dynamics of dissolved nitrogen exports from two high arctic watersheds, Melville Island, Canada[J]. Hydrology Research, 2008, 39(4): 323-335. [55] 袁晓芳, 邓娅敏, 杜尧, 等. 江汉平原高砷地下水稳定碳同位素特征及其指示意义[J]. 地质科技通报, 2020, 39(5): 156-163. doi: 10.19509/j.cnki.dzkq.2021.0008Yuan X F, Deng Y M, Du Y, et al. Characteristics of stable carbon isotopes and its implications on arsenic enrichmentin shallow groundwater of the Jianghan Plain[J]. Bulletin of Geological Science and Technology, 2020, 39(5): 156-163 (in Chinese with English abstract). doi: 10.19509/j.cnki.dzkq.2021.0008 [56] Tranvik L J, Downing J A, Cotner J B, et al. Lakes and reservoirs as regulators of carbon cycling and climate[J]. Limnology and Oceanography, 2009, 54(6): 2298-2314. [57] Bernhardt E S, Likens G E. Dissolvedorganic carbon enrichment Alters nitrogen dynamics in a forest stream[J]. Ecology, 2002, 83(6): 1689-1700. [58] Zarnetske J P, Haggerty R, Wondzell S M, et al. Labile dissolved organic carbon supply limits hyporheic denitrification[J]. Journal of Geophysical Research: Biogeosciences, 2011, 116: G04036. [59] Harms-Ringdahl P. Identifying possible sources of ammonium ions and arsenic in groundwater in the Nam Du area, Vietnam[D]. Uppsala, Swedish: Swedish University of Agricultural Sciences, 2007. [60] 吴耀国. 地下水环境中反硝化作用[J]. 环境污染治理技术与设备, 2002, 3(3): 27-31. https://www.cnki.com.cn/Article/CJFDTOTAL-HJJZ200203006.htmWu Y G. Denitrification in groundwater systems[J]. Techniques and equipment for environmental pollution control, 2002, 3(3): 27-31(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-HJJZ200203006.htm [61] Roland F A E, Darchambeau F, Borges A V, et al. Denitrification, anaerobic ammonium oxidation, and dissimilatory nitrate reduction to ammonium in an East African Great Lake (Lake Kivu)[J]. Limnology and Oceanography, 2018, 63(2): 687-701. [62] Rütting T, Boeckx P, Müller C, et al. Assessment of the importance of dissimilatory nitrate reduction to ammonium for the terrestrial nitrogen cycle[J]. Biogeosciences, 2011, 8(7): 1779-1791. [63] Buresh R J, Patrick W H. Nitratereduction to ammonium in anaerobic soil[J]. Soil Science Society of America Journal, 1978, 42(6): 913-918. [64] Hardison A K, Algar C K, Giblin A E, et al. Influence of organic carbon and nitrate loading on partitioning between dissimilatory nitrate reduction to ammonium (DNRA) and N2 production[J]. Geochimica et Cosmochimica Acta, 2015, 164: 146-160. [65] Porubsky W P, Weston N B, Joye S B. Benthic metabolism and the fate of dissolved inorganic nitrogen in intertidal sediments[J]. Estuarine, Coastal and Shelf Science, 2009, 83(4): 392-402. [66] Starr R C, Gillham R W. Denitrification andorganic carbon availability in two aquifers[J]. Ground Water, 2010, 31(6): 934-947. -





 下载:
下载: