Geochemical behavior of rare earth elements in high-temperature hot springs and its indications: A case study in the Daggyai hydrothermal area, Tibet
-
摘要:
西藏搭格架水热区位于拉萨-冈底斯地块南缘, 毗邻印度河-雅鲁藏布江缝合带中段, 区内出露酸性泉、中性泉和弱碱性泉, 为不同类型地热水中REE分布特征及其地球化学成因研究提供了理想场所。通过在搭格架采集不同类型热泉样品, 开展了样品REE浓度测试、配分模式分析和赋存形态计算, 旨在揭示高温地热环境中REE地球化学行为的指示意义。研究结果表明, 搭格架热泉中REE的地球化学行为呈非保守性, 其ΣREE浓度受热泉环境中富Fe, Al矿物(或无定形态固体)而非硫酸盐矿物的吸附过程的影响; 热泉REE配分模式和形态分布则主要受热储内氧化-还原条件及流体-岩石相互作用的控制, 是其地质成因和总体水化学特征的反映。虽然搭格架热泉中宏量组分水化学特征指示热储围岩应主要为长英质岩石, 但中、碱性热泉呈现的Ce负异常意味着热储中也可能存在碳酸盐岩。本研究为高温热泉稀土元素地球化学研究提供了典型范例。
Abstract:The Daggyai hydrothermal area (Tibet) is located on the southern margin of the Lhasa-Gangdise terrane and adjacent to the middle of the Indus-Tsangposuture. Acid, neutral, and weakly alkaline hot springs are ubiquitous in Daggyai, offering a peerless opportunity to study the distribution of rare earth elements (REE) in various geothermal waters as well as their geochemical origins. In this study, different types of the Daggyai hot springs were systematically collected to determine their REE concentrations, to discern the REE patterns and to calculate the REE speciation, which is helpful for revealing the indications of the geochemical behavior of REEs in high-temperature geothermal environments. The results of the study show that the REEs in the Daggyai hot springs behaved conservatively, with their concentrations being affected by the sorption of Fe-or Al-rich minerals or amorphous phases instead of sulfate minerals, and the REE patterns and speciation were controlled by the redox conditions and fluid-rock interactions in the reservoirs, capable of reflecting the geological genesis and the general hydrochemical characteristics of the hot springs. Although the major constituent hydrochemistry of the Daggyai hot springs demonstrates that the reservoir host rocks are primarily felsic rocks, the negative Ce anomaly of the neutral-to-alkaline hot springs implies that there are possibly carbonate rocks in the Daggyai reservoirs. This work is a typical example of relevant studies on REE geochemistry in high-temperature hot springs.
-
Key words:
- hot spring /
- rare earth element /
- water-rock interaction /
- redox condition /
- Daggyai
-
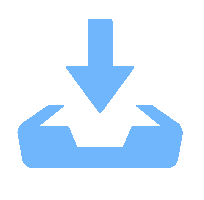
图 1 研究区地质简图及采样点分布(据文献[19]修改)
a.西藏大地构造简图; b.搭格架水热区地质简图; c.热泉采样点; d.地层剖面图; JRS.金沙江缝合带;BNS.班公错-怒江缝合带;IYZX.印度河-雅鲁藏布江缝合带;MBT.主边界逆冲断裂;Q.第四系堆积物;E3N1d.大竹卡组;E1-2d2.典中组;K1-2a1.昂仁组;KZzπBiηγ.白垩系花岗岩;1.断层;2.剖面线;3.河流
Figure 1. Simplified geological map of the study area and sampling locaitons
表 1 搭格架热泉样品主要水化学特征[21]
Table 1. Major hydrochemical characteristics of the Daggyai hot spring samples
ID pH T/℃ Eh/mV EC/(μS·cm-1) 碱度 SO4 Cl F NO3 Na K Ca Mg Fe Si B Li Rb Cs 阴阳离子平衡相对误差/% ρB/(mg·L-1) ρB/(μg·L-1) DG00 8.59 79.3 -210 1 873.0 670 85.5 155.50 24.50 1.86 453.90 52.90 43.10 0.01 0.15 137.9 102.2 5 427 654.0 6 591 7.8 DG02 3.00 36.7 447 439.8 0 104.7 1.68 0.37 2.01 6.29 3.66 9.57 0.90 2.03 85.0 1.16 168 82.6 1 295 6.9 DG03 4.46 69.1 -12 386.3 0 144.9 1.32 0.17 2.66 8.44 2.38 20.20 1.32 0.08 31.1 1.09 78.8 47.9 571 -5.1 DG05 8.25 78.9 -233 2 004.0 655 90.1 162.30 26.30 1.88 482.40 56.90 33.90 0.01 0.22 167.7 106.9 5 749 668 6 567 9.3 DG07 6.97 82.1 -225 1 872.0 595 88.0 148.70 24.10 1.85 451.20 53.00 23.50 0.05 0.15 151.6 103.40 5 736 662.0 6 753 9.2 DG08 6.92 75.5 -235 1 994.0 625 84.8 155.30 24.80 n.d. 436.50 51.20 7.16 0.03 0.03 151.3 98.80 5 793 653.0 6 680 3.9 DG09 6.90 41.5 -215 1 678.0 555 76.2 147.90 26.20 1.88 411.00 43.90 21.10 0.05 0.09 121.1 97.70 5 112 521.0 5 775 6.5 DG11 6.99 79.9 -324 1 966.0 625 86.6 159.40 25.80 2.06 445.50 52.10 15.20 0.02 0.08 153.5 101.40 5 001 580.0 5 575 4.9 DG12 7.00 77.8 -293 1 805.0 550 77.6 144.80 21.70 n.d. 407.70 46.00 6.12 0.01 0.01 126.2 96.80 4 816 533.0 5 618 5.8 注:n.d.代表未检出 表 2 搭格架热泉稀土元素地球化学特征
Table 2. Geochemical characteristics of REEs in the Daggyai hot springs
DG00 DG02 DG03 DG05 DG07 DG08 DG09 DG11 DG12 La 0.64 0.53 0.62 0.64 0.62 0.36 0.58 0.37 0.33 Ce 1.18 1.08 1.16 1.05 0.97 0.64 0.82 0.62 0.52 Pr 0.14 0.12 0.13 0.14 0.10 0.08 0.13 0.07 0.07 Nd 0.65 0.51 0.61 0.41 0.38 0.33 0.38 0.28 0.25 Sm 0.19 0.18 0.20 0.16 0.19 0.11 0.16 0.11 0.15 Eu 0.05 0.02 0.07 0.04 0.05 0.03 0.04 0.03 0.04 Gd 0.13 0.14 0.19 0.16 0.10 0.05 0.12 0.07 0.08 Tb 0.02 0.03 0.03 0.02 0.03 0.01 0.02 0.01 0.01 Dy ρB/(μg·L-1) 0.14 0.15 0.16 0.16 0.13 0.08 0.11 0.06 0.05 Ho 0.04 0.04 0.03 0.02 0.04 0.01 0.03 0.02 0.02 Er 0.06 0.09 0.12 0.09 0.07 0.04 0.06 0.06 0.06 Tm 0.02 0.02 0.02 0.02 0.02 0.02 0.01 0.02 0.02 Yb 0.09 0.15 0.08 0.10 0.09 0.07 0.07 0.05 0.08 Lu 0.02 0.01 0.01 0.02 0.02 0.03 0.01 0.02 0.01 Y 0.56 0.68 0.78 0.54 0.42 0.23 0.39 0.33 0.21 LREE 2.85 2.45 2.80 2.44 2.31 1.55 2.11 1.48 1.35 HREE 0.52 0.62 0.64 0.58 0.50 0.30 0.43 0.30 0.32 ΣREE 3.93 3.75 4.22 3.56 3.23 2.07 2.93 2.11 1.89 Ce/Ce* 0.86 0.92 0.88 0.76 0.85 0.83 0.66 0.82 0.76 Pr/Pr* 0.97 1.04 0.97 1.36 0.97 1.06 1.43 1.06 1.15 Eu/Eu* 1.55 0.68 1.61 1.20 1.47 1.58 1.34 1.66 1.45 -
[1] Munemoto T, Ohmori K, Iwatsuki T. Rare earth elements (REE) in deep groundwater from granite and fracture-filling calcite in the Tono area, central Japan: Prediction of REE fractionation in paleo- to present-day groundwater[J]. Chemical Geology, 2015, 417: 58-67. doi: 10.1016/j.chemgeo.2015.09.024 [2] Bau M. Scavenging of dissolved yttrium and rare earths by precipitating iron oxyhydroxide: Experimental evidence for Ce oxidation, Y-Ho fractionation, and lanthanidetetrad effect[J]. Geochimica et Cosmochimica Acta, 1999, 63: 67-77. doi: 10.1016/S0016-7037(99)00014-9 [3] Sholkovitz E R. Chemical evolution of rare earth elements: Fractionation between colloidal and solution phases of filtered river water[J]. Earth and Planetary Science Letters, 1992, 114: 77-84. doi: 10.1016/0012-821X(92)90152-L [4] Tweed S O, Weaver T R, Cartwright I, et al. Behavior of rare earth elements in groundwater during flow andmixing in fractured rock aquifers: An example from the Dandenong ranges, Southeast Australia[J]. Chemical Geology, 2006, 234: 291-307. doi: 10.1016/j.chemgeo.2006.05.006 [5] Michard A. Rare earth systematics in hydrothermal fluids[J]. Geochimica et Cosmochimica Acta, 1989, 53: 745-750. doi: 10.1016/0016-7037(89)90017-3 [6] Johannesson K H, Farnham I M, Guo C, et al. Rare earth element fractionation and concentration variations along a groundwater flow path within a shallow, basin-fill aquifer, southern Nevada, USA[J]. Geochimica et Cosmochimica Acta, 1999, 63: 2697-2708. doi: 10.1016/S0016-7037(99)00184-2 [7] Noack C W, Dzombak D A, Karamalidis A K. Rare earth element distributions and trends in natural waters with a focus on groundwater[J]. Environmental Science and Technology, 2014, 48: 4317-4326. doi: 10.1021/es4053895 [8] 王雨婷, 李俊霞, 薛肖斌, 等. 华北平原与大同盆地原生高碘地下水赋存主控因素的异同[J]. 地球科学, 2021, 46(1): 308-320. https://www.cnki.com.cn/Article/CJFDTOTAL-DQKX202101024.htmWang Y T, Li J X, Xue X B, et al. Similarities and differences of main controlling factors of natural high iodine groundwater between North China Plain and Datong Basin[J]. Earth Science, 2021, 46(1): 308-320(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-DQKX202101024.htm [9] Zhang J, Nozaki Y. Behavior of rare earth elements in seawater at the ocean margin: A study along the slopes of the Sagami and Nankai troughs near Japan[J]. Geochimica et Cosmochimica Acta, 1998, 62: 1307-1317. doi: 10.1016/S0016-7037(98)00073-8 [10] Craddock P R, Bach W, Seewald J S, et al. Rare earth element abundances in hydrothermal fluids from the Manus Basin, Papua New Guinea: Indicators of subseafloor hydrothermal processes in back- arc basins[J]. Geochimica et Cosmochimica Acta, 2010, 74: 675-683. [11] Deluca F, Mongelli G, Paternoster M, et al. Rare earth elements distribution and geochemical behaviour in the volcanic groundwaters of Mount Vulture, southern Italy[J]. Chemical Geology, 2020, 539: 119503. doi: 10.1016/j.chemgeo.2020.119503 [12] Klinkhammer G P, Elderfield H, Edmond J M, et al. Geochemical implications of rare earth element patterns in hydrothermal fluids from mid-ocean ridges[J]. Geochimica et Cosmochimica Acta, 1994, 58: 5105-5113. doi: 10.1016/0016-7037(94)90297-6 [13] Dídac N, Esteve C, Carmen G, et al. REE and Sm-Nd clues of high-temperature fluid-rock interaction in the Riópar dolomitization (SE Spain)[J]. Procedia Earth and Planetary Science, 2017, 17: 448-451. doi: 10.1016/j.proeps.2016.12.113 [14] Wan Y, Wang X, Chou I, et al. Role of sulfate in the transport and enrichment of REE in hydrothermal systems[J]. Earth and Planetary Science Letters, 2021, 569: 117068. doi: 10.1016/j.epsl.2021.117068 [15] Li Z, Zhang S, Zheng Y, et al. Mobilization and fractionation of HFSE and REE by high fluorine fluid of magmatic origin during the alteration of amphibolite[J]. Lithos, 2022, 420/421: 106701. doi: 10.1016/j.lithos.2022.106701 [16] Gob S, Loges A, Nolde N, et al. Major and trace element compositions (including REE) of mineral, thermal, mine and surface waters in SW Germany and implications for water-rock interaction[J]. Applied Geochemistry, 2013, 33: 127-152. doi: 10.1016/j.apgeochem.2013.02.006 [17] Oliveri Y, Cangemi M, Capasso G, et al. Pathways and fate of REE in the shallow hydrothermal aquifer of Vulcano Island (Italy)[J]. Chemical Geology, 2019, 512: 121-129. doi: 10.1016/j.chemgeo.2019.02.037 [18] Bai D, Unsworth M J, Meju M A, et al. Crustal deformation of the eastern Tibetan Plateau revealed by magnetotelluric imaging[J]. Nature Geoscience, 2010, 3: 358-362. doi: 10.1038/ngeo830 [19] 王鹏. 藏南碰撞造山带典型水热区现代地球化学过程与小流域CO2源、汇关系研究[D]. 重庆: 西南大学, 2013.Wang P. Study on modern geochemical processes and CO2 source and sink relationship of small watershed of typical hydrothermal area in southern Tibet collision orogenic belt[D]. Chongqing: Southwest University, 2013(in Chinese with English abstract). [20] Hoke L, Lamb S, Hilton D R, et al. Southern limit of mantle-derived geothermal helium emissions in Tibet: Implications for lithospheric structure[J]. Earth Planet Science Letter., 2000, 180(3): 297-308. [21] Liu M, Guo Q, Wu G, et al. Boron geochemistry of the geothermal waters from two typical hydrothermal systems in southern Tibet (China): Daggyai and Quzhuomu[J]. Geothermics, 2019, 82: 190-202. doi: 10.1016/j.geothermics.2019.06.009 [22] Giggenbach W. Geothermal solute equilibria: Derivation of Na-K-Mg-Ca geoindicators[J]. Geochemica et Cosmochimica Acta, 1988, 52, 2749-2765. doi: 10.1016/0016-7037(88)90143-3 [23] Santos-Raga G, Santoyo E, Guevara M, et al. Tracking geochemical signatures of rare earth and trace elements in spring waters and outcropping rocks from the hidden geothermal system of Acoculco, Puebla (Mexico)[J]. Journal of Geochemical Exploration, 2021, 227: 106798. doi: 10.1016/j.gexplo.2021.106798 [24] Shakeri A, Ghoreyshinia S, Mehrabi B, et al. Rare earth elements geochemistry in springs from Taftan geothermal area SE Iran[J]. Journal of Volcanology & Geothermal Research, 2015, 304: 49-61. [25] 赵振华, 王一先, 钱志鑫, 等. 西藏南部花岗岩类稀土元素地球化学[J]. 地球化学, 1981, 10(1): 26-35. doi: 10.3321/j.issn:0379-1726.1981.01.004Zhao Z H, Wang Y X, Qian Z X, et al. REE geochemistry of granitoids in southen Tibet[J]. Geochimica, 1981, 10(1): 26-35(in Chinese with English abstract). doi: 10.3321/j.issn:0379-1726.1981.01.004 [26] Smedley P L. The geochemistry of rare earth elements in groundwater from the Carnmenellis area, southwest England[J]. Geochimica et Cosmochimica Acta, 1991, 55: 2767-2779. doi: 10.1016/0016-7037(91)90443-9 -





 下载:
下载: