Kinetic characteristics of methane hydrate in functionalized multi-walled carbon nanotubes and L-leucine compounding system
-
摘要:
加快天然气水合物形成, 对基于水合物法的天然气储运、气体分离和二氧化碳捕集技术的推动具有重要意义。采用恒温恒容法研究了
w B=0.05%功能化(羟基化、羧基化和氨基化)多壁碳纳米管和w B=1.0% L-亮氨酸复配体系中甲烷水合物动力学特征。研究表明, 多壁碳纳米管、羧基化和羟基化多壁碳纳米管与L-亮氨酸的复配, 可使甲烷水合物诱导成核时间大幅缩短至25, 22, 13 min左右, 促进效果与典型促进剂十二烷基硫酸钠相当, 且促进效果优于单一添加剂体系。复配体系甲烷储气质量分数具有良好表现, 可达136~142 mg/g。对甲烷平均吸收速率和瞬时吸收速率的分析表明, 多壁碳纳米管对生长阶段甲烷水合物的生长动力学影响很小。复配体系和L-亮氨酸体系中甲烷水合物的生长具有相似性, 均呈现出甲烷气体吸收速率快速增加到最大值, 然后迅速下降并完成生长的特点。综合分析表明, 多壁碳纳米管和L-亮氨酸的复配对甲烷水合物的成核速率具有协同增强效应, 而生长阶段的进程与速率主要受L-亮氨酸影响。该研究为探索不同类型添加剂在强化甲烷水合物生成动力学上的差异化机理提供了新思路。Abstract:Objective Accelerated the generation of natural gas hydrate is crucial for advancing hydrate-based technologies such as gas storage, gas separation, and CO2 capture.
Methods The kinetic characteristics of methane hydrate generated with the
w B=0.05% functionalized (hydroxylated, carboxylated, and aminated) multi-walled carbon nanotubes(MWCNT) system, and in combination with thew B=1.0% L-leucine were investigated through constant temperature and constant volume methods.Results The combination of multiwalled carbon nanotubes and carboxylated and hydroxylated multiwalled carbon nanotubes with L-leucine, significantly reduced the induction time for natural gas hydrate nucleation to approximately 25, 22, and 13 minutes, respectively. This promotion effect is comparable to that of the typical promoter sodium dodecyl sulfate, and the promotion effect is better than that of a single additive system. The methane storage density of the compounded system reached 136-142 mg/g. Analysis of both the average and instantaneous methane uptake rates indicated that multiwalled carbon nanotubes had minimal impact on the growth kinetics of methane hydrate during the growth phase. The growth of methane hydrate in both the compounded and L-leucine systems were similar, characterized by a rapid increase in uptake rate to a peak value, followed by a rapid decrease and eventual completion of the growth phase.
Conclusion A comprehensive analysis suggests that the combination of MWCNTs and L-leucine synergistically enhances the nucleation rate of methane hydrate, whereas the process and rate of the growth phase are predominantly influenced by L-leucine. This study presents a new idea for exploring the differentiation mechanism of different types of additives in enhancing the kinetics of methane hydrate generation.
-
Key words:
- methane hydrate /
- multi-walled carbon nanotube /
- L-leucine /
- synergistic promotion /
- nucleation /
- growth
-
-
[1] SLOAN E D, KOH C A. Clathrate hydrates of natural gases[M]. Boca Raton, FL: CRC Press, 2008. [2] BOSWELL R, COLLETT T S. Current perspectives on gas hydrate resources[J]. Energy & Environmental Science, 2011, 4(4): 1206-1215. [3] 郁桂刚, 欧文佳, 吴翔, 等. 天然气水合物分解动力学研究进展[J]. 地质科技通报, 2023, 42(3): 175-188. doi: 10.19509/j.cnki.dzkq.tb20210668YU G G, OU W J, WU X, et al. Research advances on the dissociation dynamics of natural gas hydrates[J]. Bulletin of Geological Science and Technology, 2023, 42(3): 175-188. (in Chinese with English abstract) doi: 10.19509/j.cnki.dzkq.tb20210668 [4] 宁伏龙, 方翔宇, 李彦龙, 等. 天然气水合物开采储层出砂研究进展与思考[J]. 地质科技通报, 2020, 39(1): 137-148. doi: 10.19509/j.cnki.dzkq.2020.0115NING F L, FANG X Y, LI Y L, et al. Research status and perspective on wellbore sand production from hydrate reservoirs[J]. Bulletin of Geological Science and Technology, 2020, 39(1): 137-148. (in Chinese with English abstract) doi: 10.19509/j.cnki.dzkq.2020.0115 [5] VELUSWAMY H P, WONG A J H, BABU P, et al. Rapid methane hydrate formation to develop a cost effective large scale energy storage system[J]. Chemical Engineering Journal, 2016, 290: 161-173. doi: 10.1016/j.cej.2016.01.026 [6] BABU P, NAMBIAR A, HE T B, et al. A review of clathrate hydrate based desalination to strengthen energy-water nexus[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(7): 8093-8107. [7] LÜ Y N, WANG S S, SUN C Y, et al. Desalination by forming hydrate from brine in cyclopentane dispersion system[J]. Desalination, 2017, 413: 217-222. doi: 10.1016/j.desal.2017.03.025 [8] BABU P, LINGA P, KUMAR R, et al. A review of the hydrate based gas separation(HBGS) process for carbon dioxide pre-combustion capture[J]. Energy, 2015, 85: 261-279. doi: 10.1016/j.energy.2015.03.103 [9] LIU Y, GUO K H, LIANG D Q, et al. Effects of magnetic fields on HCFC-141b refrigerant gas hydrate formation[J]. Science in China Series B(Chemistry), 2003, 46(4): 407-415. [10] 巫术胜, 肖睿, 黄冲, 等. 四丁基溴化铵水合物在空调蓄冷中的应用研究[J]. 制冷学报, 2006, 27(6): 48-51.WU S S, XIAO R, HUANG C, et al. Research on clathrate hydrate of tetra-n-butylammonium bromide as cold-storage material in air-conditioning[J]. Journal of Refrigeration, 2006, 27(6): 48-51. (in Chinese with English abstract) [11] ZHENG J J, CHONG Z R, QURESHI M F, et al. Carbon dioxide sequestration via gas hydrates: A potential pathway toward decarbonization[J]. Energy & Fuels, 2020, 34(9): 10529-10546. [12] MIMACHI H, TAKAHASHI M, TAKEYA S, et al. Effect of long-term storage and thermal history on the gas content of natural gas hydrate pellets under ambient pressure[J]. Energy & Fuels, 2015, 29(8): 4827-4834. [13] VELUSWAMY H P, KUMAR A, SEO Y, et al. A review of solidified natural gas(SNG) technology for gas storage via clathrate hydrates[J]. Applied Energy, 2018, 216: 262-285. [14] 杨亮. 甲烷水合物生成的静态强化技术[D]. 广州: 华南理工大学, 2013.YANG L. Static enhancement technology of methane hydrate formation[D]. Guangzhou: South China University of Technology, 2013. (in Chinese with English abstract) [15] OHMURA R, KASHIWAZAKI S, SHIOTA S, et al. Structure-I and structure-H hydrate formation using water spraying[J]. Energy & Fuels, 2002, 16(5): 1141-1147. [16] 邵子越, 申小冬, 李延霞, 等. 生物胶对二氧化碳水合物生成动力学影响实验研究[J]. 低碳化学与化工, 2023, 48(2): 155-161.SHAO Z Y, SHEN X D, LI Y X, et al. Experimental study of influence of biological gums on formation kinetics of carbon dioxide hydrates[J]. Low-Carbon Chemistry and Chemical Engineering, 2023, 48(2): 155-161. (in Chinese with English abstract) [17] KUMAR A, BHATTACHARJEE G, KULKARNI B D, et al. Role of surfactants in promoting gas hydrate formation[J]. Industrial & Engineering Chemistry Research, 2015, 54(49): 12217-12232. [18] WANG F, LIU G Q, MENG H L, et al. Improved methane hydrate formation and dissociation with nanosphere-based fixed surfactants As promoters[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(4): 2107-2113. [19] LO C, ZHANG J S, SOMASUNDARAN P, et al. Investigations of surfactant effects on gas hydrate formation via infrared spectroscopy[J]. Journal of Colloid and Interface Science, 2012, 376(1): 173-176. [20] BHATTACHARJEE G, LINGA P. Amino acids as kinetic promoters for gas hydrate applications: A mini review[J]. Energy & Fuels, 2021, 35(9): 7553-7571. [21] VELUSWAMY H P, KUMAR A, KUMAR R, et al. An innovative approach to enhance methane hydrate formation kinetics with leucine for energy storage application[J]. Applied Energy, 2017, 188: 190-199. [22] LIU Y, CHEN B Y, CHEN Y L, et al. Methane storage in a hydrated form as promoted by leucines for possible application to natural gas transportation and storage[J]. Energy Technology, 2015, 3(8): 815-819. [23] SHANKER PANDEY J, JOULJAMAL DAAS Y, PAUL KARCZ A, et al. Methane hydrate formation behavior in the presence of selected amino acids[J]. Journal of Physics(Conference Series), 2020, 1580(1): 012003. [24] VELUSWAMY H P, LEE P Y, PREMASINGHE K, et al. Effect of biofriendly amino acids on the kinetics of methane hydrate formation and dissociation[J]. Industrial & Engineering Chemistry Research, 2017, 56(21): 6145-6154. [25] PRASAD P S R, SAI KIRAN B. Clathrate hydrates of greenhouse gases in the presence of natural amino acids: Storage, transportation and separation applications[J]. Scientific Reports, 2018, 8(1): 8560. [26] NASHED O, PARTOON B, LAL B, et al. Review the impact of nanoparticles on the thermodynamics and kinetics of gas hydrate formation[J]. Journal of Natural Gas Science and Engineering, 2018, 55: 452-465. [27] RAHMATI-ABKENAR M, MANTEGHIAN M, PAHLAVANZADEH H. Experimental and theoretical investigation of methane hydrate induction time in the presence of triangular silver nanoparticles[J]. Chemical Engineering Research and Design, 2017, 120: 325-332. [28] PAHLAVANZADEH H, REZAEI S, KHANLARKHANI M, et al. Kinetic study of methane hydrate formation in the presence of copper nanoparticles and CTAB[J]. Journal of Natural Gas Science and Engineering, 2016, 34: 803-810. [29] ALIABADI M, RASOOLZADEH A, ESMAEILZADEH F, et al. Experimental study of using CuO nanoparticles as a methane hydrate promoter[J]. Journal of Natural Gas Science and Engineering, 2015, 27: 1518-1522. [30] ABDI-KHANGHAH M, ADELIZADEH M, NASERZADEH Z, et al. Methane hydrate formation in the presence of ZnO nanoparticle and SDS: Application to transportation and storage[J]. Journal of Natural Gas Science and Engineering, 2018, 54: 120-130. [31] CHARI V D, SHARMA D V S G K, PRASAD P S R, et al. Methane hydrates formation and dissociation in nano silica suspension[J]. Journal of Natural Gas Science and Engineering, 2013, 11: 7-11. [32] PARK S S, LEE S B, KIM N J. Effect of multi-walled carbon nanotubes on methane hydrate formation[J]. Journal of Industrial and Engineering Chemistry, 2010, 16(4): 551-555. [33] GOVINDARAJ V, MECH D, PANDEY G, et al. Kinetics of methane hydrate formation in the presence of activated carbon and nano-silica suspensions in pure water[J]. Journal of Natural Gas Science and Engineering, 2015, 26: 810-818. [34] KIM N J, PARK S S, KIM H T, et al. A comparative study on the enhanced formation of methane hydrate using CM-95 and CM-100 MWCNTs[J]. International Communications in Heat and Mass Transfer, 2011, 38(1): 31-36. [35] WANG F, LUO S J, FU S F, et al. Methane hydrate formation with surfactants fixed on the surface of polystyrene nanospheres[J]. Journal of Materials Chemistry A, 2015, 3(16): 8316-8323. [36] KAKATI H, MANDAL A, LAIK S. Promoting effect of Al2O3/ZnO-based nanofluids stabilized by SDS surfactant on CH4++C2H6+C3H8 hydrate formation[J]. Journal of Industrial and Engineering Chemistry, 2016, 35: 357-368. [37] WANG F, MENG H L, GUO G, et al. Methane hydrate formation promoted by-SO3--coated graphene oxide anosheets[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(8): 6597-6604. [38] 张雪艳, 周诗岽, 姬浩洋, 等. 氧化石墨烯/纳米石墨颗粒与SDS复配对CO2水合物生成特性的影响[J]. 天然气化工(C1化学与化工), 2021, 46(2): 53-58.ZHANG X Y, ZHOU S D, JI H Y, et al. Effect of GO/GN and SDS compound system on formation characteristics of CO2 hydrate[J]. Natural Gas Chemical Industry(C1 Chemistry and Chemical Engineering), 2021, 46(2): 53-58. (in Chinese with English abstract) [39] PENG D Y, ROBINSON D B. A new two-constant equation of state[J]. Industrial & Engineering Chemistry Fundamentals, 1976, 15(1): 59-64. [40] CASCO M E, SILVESTRE-ALBERO J, RAMÍREZ-CUESTA A J, et al. Methane hydrate formation in confined nanospace can surpass nature[J]. Nature Communications, 2015, 6: 6432. [41] DENNING S, MAJID A A A, LUCERO J M, et al. Methane hydrate growth promoted by microporous zeolitic imidazolate frameworks ZIF-8 and ZIF-67 for enhanced methane storage[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(27): 9001-9010. [42] NGUYEN N N, NGUYEN A V. Hydrophobic effect on gas hydrate formation in the presence of additives[J]. Energy & Fuels, 2017, 31: 10311-10323. -
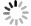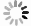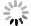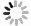




 下载:
下载: