Exploring the feasibility and influencing factors of phosphorus recovery from phosphorus-rich groundwater based on struvite precipitation methods
-
摘要:
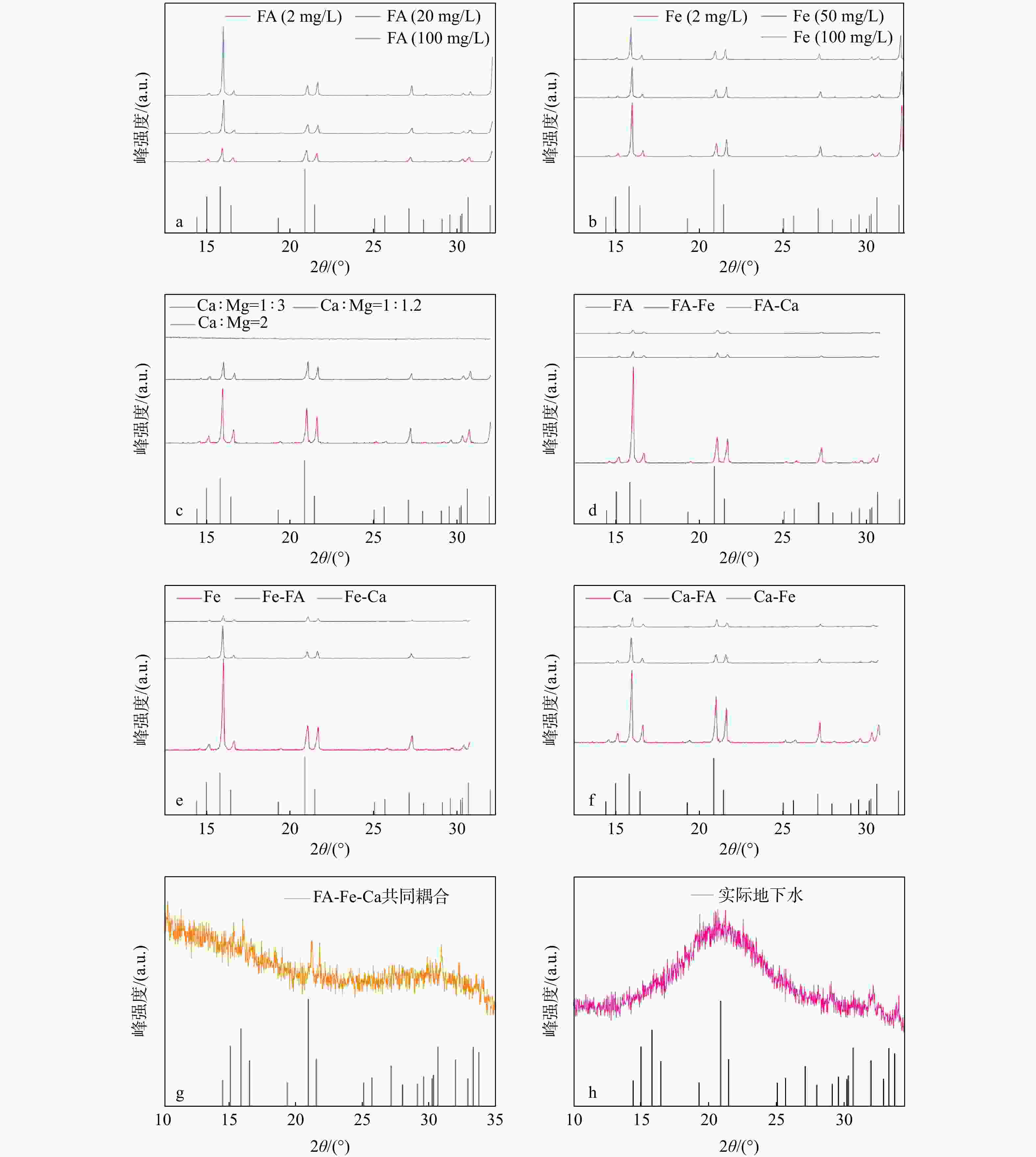
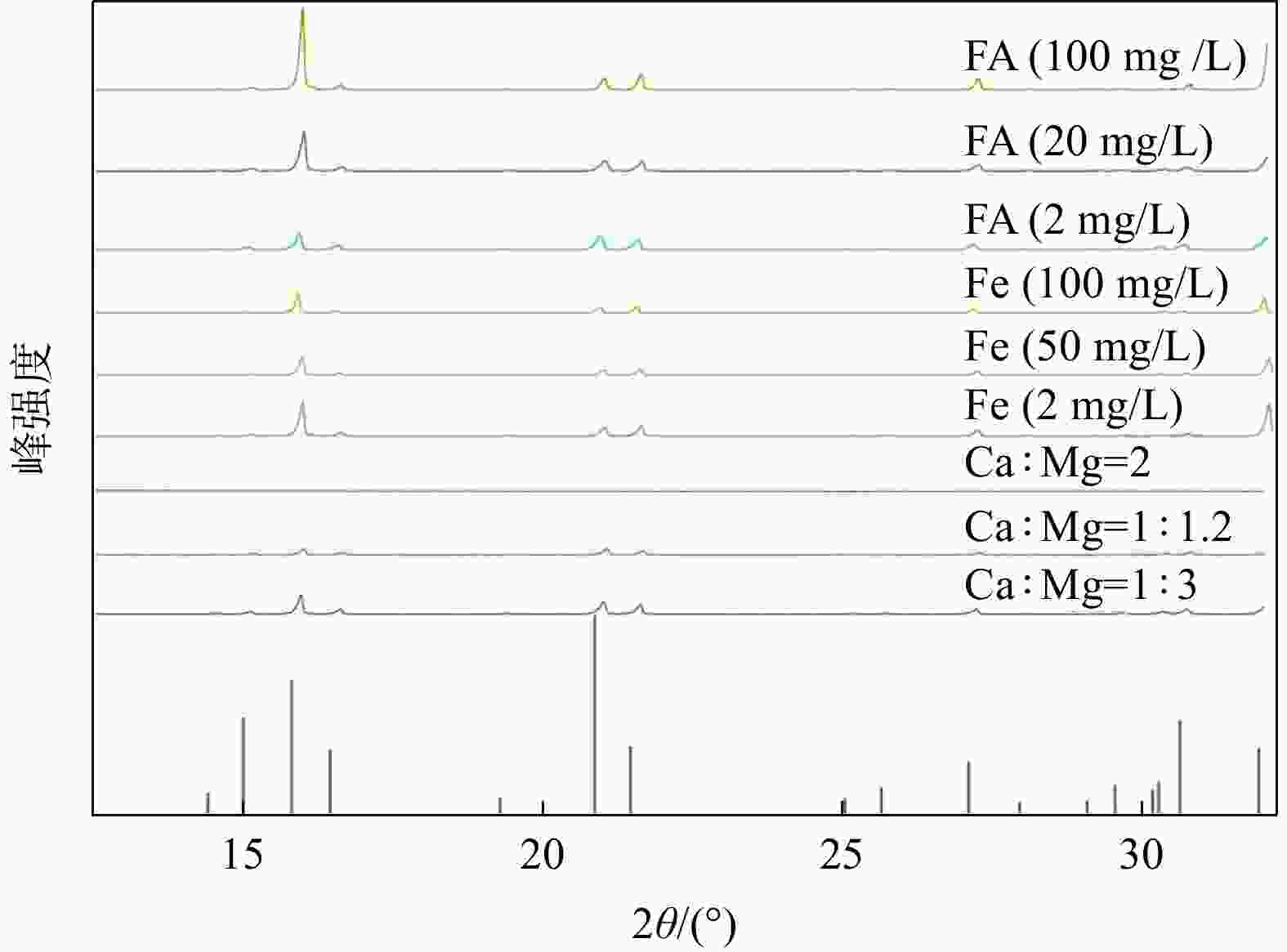
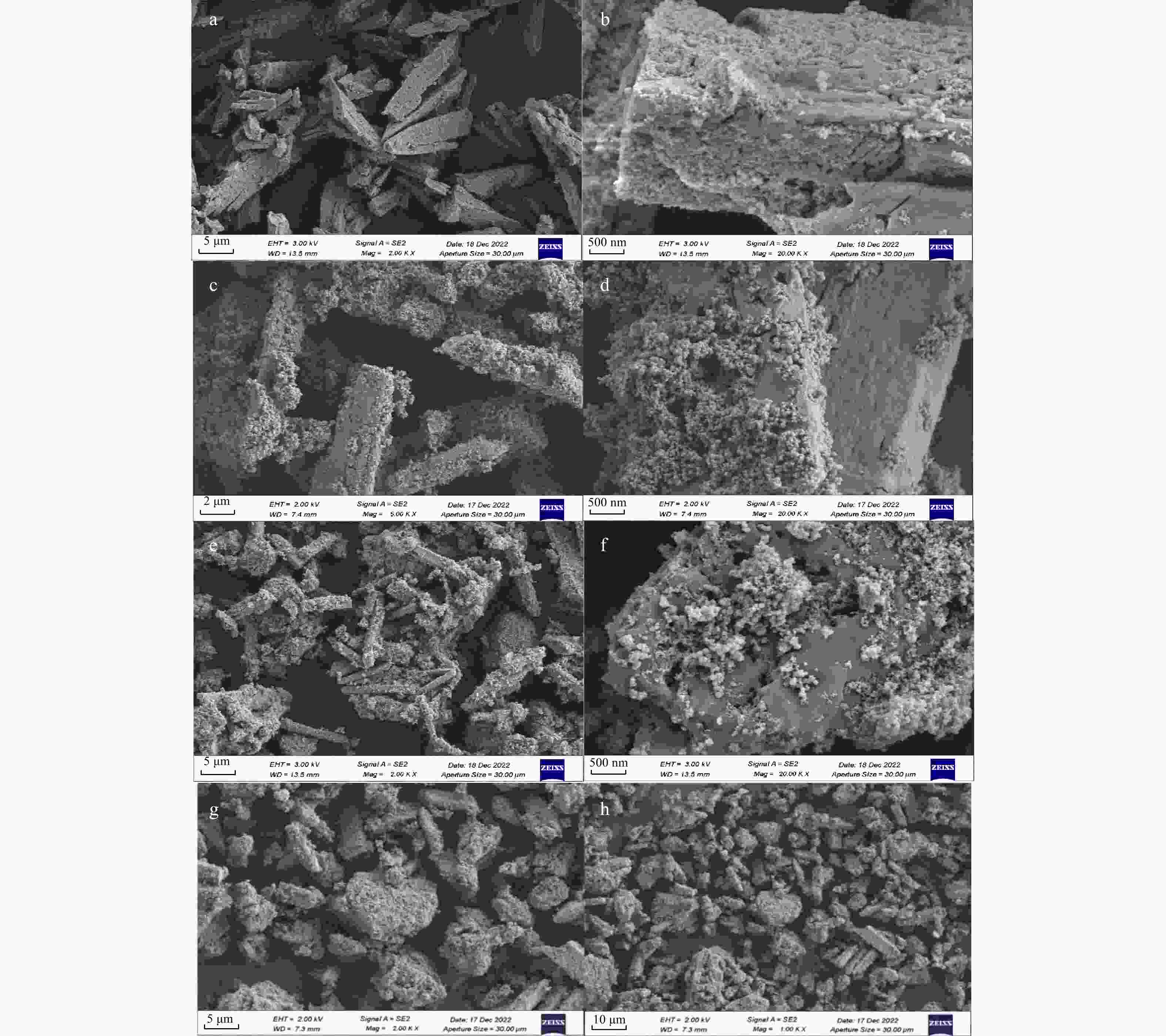
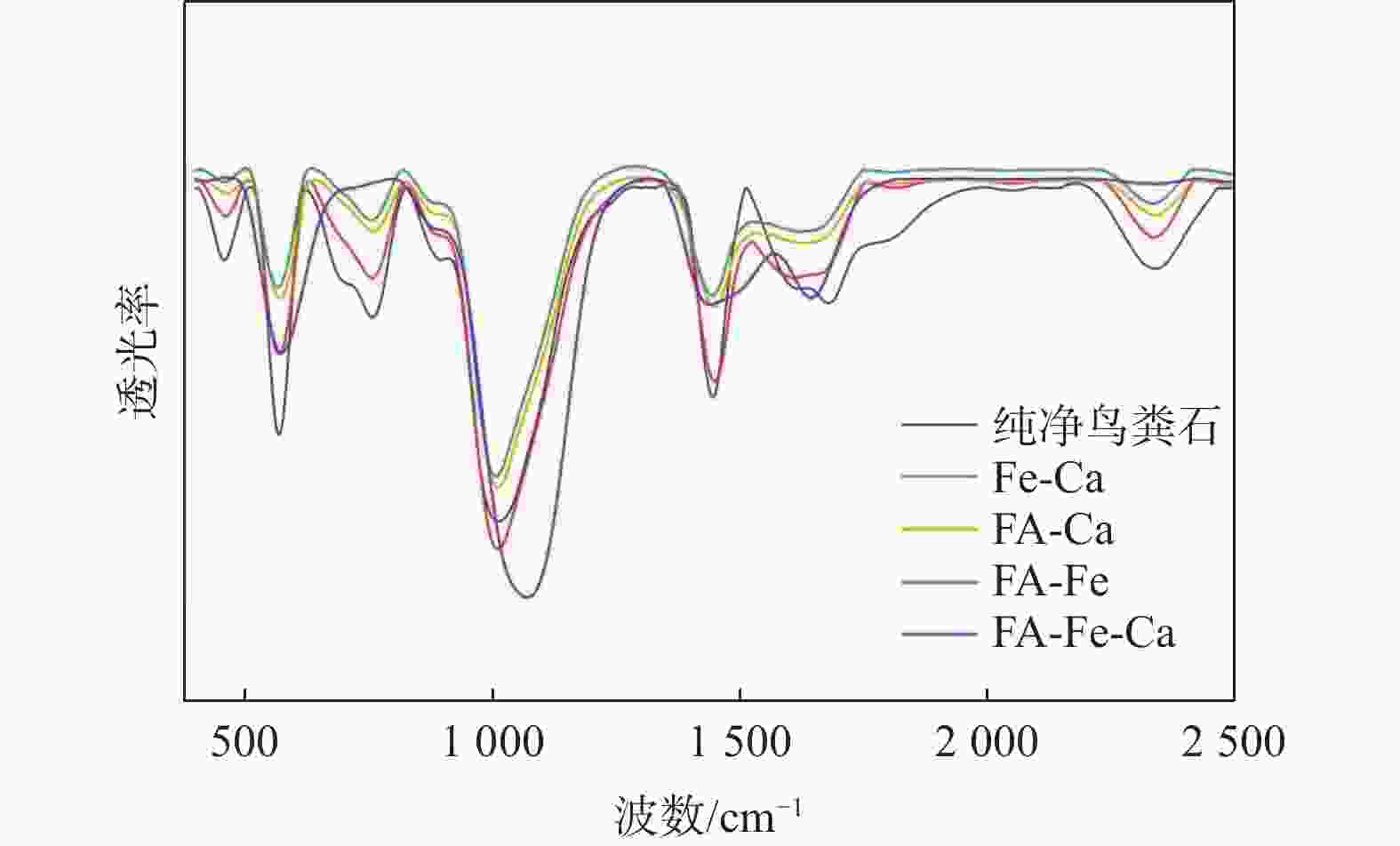
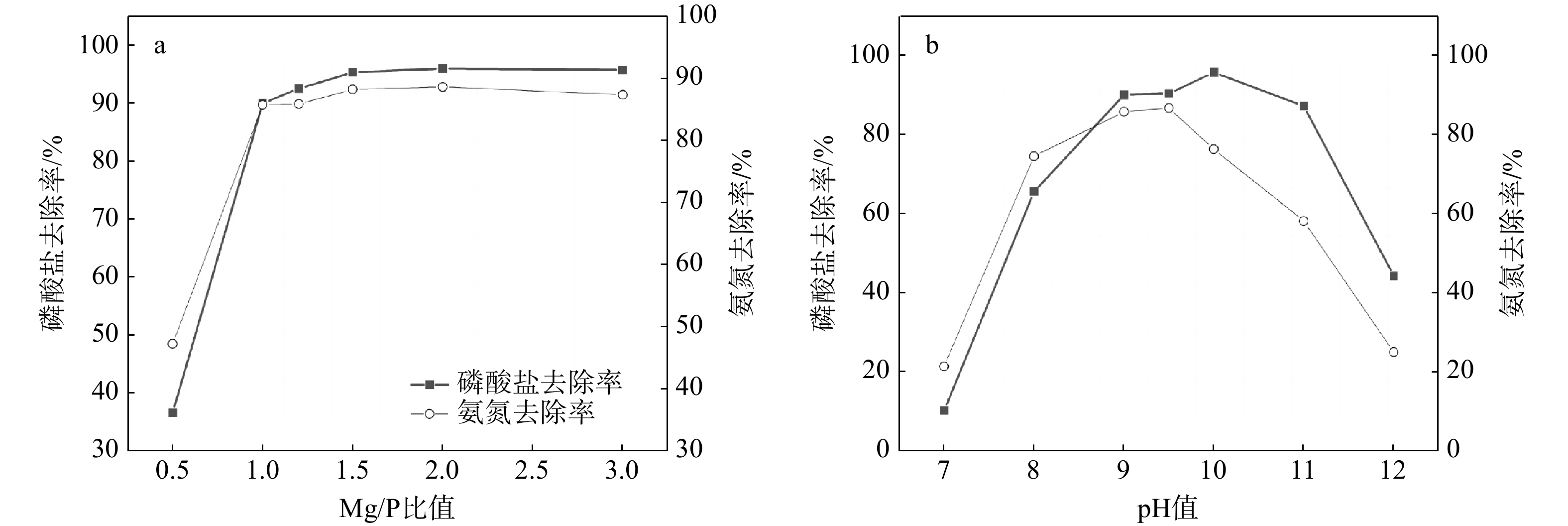
磷供应短缺和水体磷污染已成为全球性危机。鸟粪石沉淀法是最为经济有效的磷回收方法,其磷的回收率可以达到95%以上,目前已广泛应用于污水中磷的资源化。天然富磷地下水近年来备受关注,但目前尚未有基于鸟粪石法开展富磷地下水中磷回收的报道。探讨了在富磷、富钙、富铁、富黄腐酸(FA)地下水中利用鸟粪石法在pH值为9.5的环境下回收磷的影响因素与可行性。利用X射线衍射(XRD)、扫描电镜(SEM)和傅里叶变换红外光谱(FTIR)等方法对人工合成地下水与天然地下水开展了研究。结果表明,随着钙浓度增加沉淀中鸟粪石纯度迅速下降到10%以下,XRD图谱中鸟粪石的峰消失,SEM图谱中鸟粪石表面被无定形磷酸钙覆盖,单独添加铁和黄腐酸后鸟粪石的纯度变化较小,SEM图谱显示固体表面出现絮状沉淀。影响因子共存条件下得到的鸟粪石沉淀X射线光谱显示出无规则峰值,傅里叶红外光谱图分别在波数453,720,750,
1608 ,1679 cm−1处的峰消失,表明高浓度的钙能显著抑制地下水中鸟粪石的形成,铁和黄腐酸对鸟粪石形成的影响相对较弱;研究因子的共存会加剧抑制鸟粪石的形成,3个因子共同决定了鸟粪石能否在地下水中有效沉淀。本研究识别了鸟粪石沉淀法回收地下水中磷的影响因素与机制,研究结果将有助于富磷地下水中磷回收策略的制定。Abstract:Objective The global phosphorus (P) supply shortage and water pollution crisis necessitate an urgent shift from simply removing polluted phosphorus to leveraging it as a resource. Among recovery methods, the struvite precipitation method is recognized for its cost-effectiveness and high efficiency, achieving phosphorus recovery rate exceeding 95%. This method has been widely used in the reclamation of phosphorus in sewage. Despite extensive research on naturally P-rich groundwater in recent years, there is a lack of studies focusing on phosphorus recovery using the struvite method.
Methods This study explores the influencing factors and feasibility of employing the struvite method to recover phosphorus from groundwater abundant in phosphorus, calcium, iron, and fulvic acid (FA) at an optimal pH of 9.5. The aim is to develop an integrated phosphorus recycling system for P-rich groundwater and offer constructive suggestions for groundwater phosphorus recycling. Advanced techniques such as X-ray diffraction (XRD), scanning electron microscopy (SEM), and Fourier transform infrared (FTIR) spectroscopy were utilized alongside the molybdenum blue method for phosphoric acid detection, the Nash reagent was used for ammonia nitrogen detection, and Origin 9.0 was used for data visualization. These techniques have been used to thoroughly study both synthetic and natural groundwater.
Results The results show that as calcium concentration increases, the purity of struvite declines rapidly to below 10%, with the struvite peak vanishing in XRD patterns and amorphous calcium phosphate covering struvite surface in SEM patterns. When iron and/or fulvic acid were added individually, the struvite purity remained relatively unchanged. The XRD patterns revealed a weakened struvite peak, while the SEM patterns showed that flocculation precipitation occurred on the solid surface. The X-ray spectra of struvite precipitates obtained under the coexistence of influencing factors showed irregular peaks, with the peaks at 453 cm−1, 720 cm−1, 750 cm−1,
1608 cm−1, and1679 cm−1 disappearing from the FTIR spectra. These results suggest that high calcium ion concentrations significantly inhibit struvite formation in groundwater, whereas iron ions and fulvic acid have minor effects. The coexistence of these factors intensifies the inhibition of struvite formation, ultimately determining whether struvite can be effectively precipitated in groundwater.Conclusion This study identifies the key factors and mechanisms affecting phosphoric recovery from groundwater through struvite precipitation. The insights gained from this research are valuable for formulating effective recovery strategies for phosphorus in P-rich groundwater.
-
Key words:
- struvite /
- phosphorus recovery /
- groundwater /
- calcium ion /
- iron ion
-
表 1 不同干扰因素、各干扰因素耦合及地下水实际情况下产生的沉淀物的纯度和粒径
Table 1. Purity and particle size of precipitates generated in the presence of different interfering factors, combination of interfering factors, and actual groundwater
干扰因素 鸟粪石纯度/% 鸟粪石粒径/nm Ca/Mg 1:5 83.45 51.10 1:3 73.88 57.59 1∶1.2 52.22 54.52 2∶1 14.52 57.52 Fe 10 mg/L 91.50 50.04 50 mg/L 77.52 48.27 100 mg/L 44.10 50.65 FA 5 mg/L 74.68 50.52 20 mg/L 78.96 51.12 50 mg/L 72.98 62.43 FA-Fe 耦合 55.98 50.52 FA-Ca 耦合 48.52 60.10 Ca-Fe耦合 43.56 62.50 FA-Fe-Ca 耦合 16.42 63.26 天然地下水 88.0 69.50 -
[1] 王焰新,杜尧,邓娅敏,等,湖底地下水排泄与湖泊水质演化[J]. 地质科技通报,2022,41(1):1-10.WANG Y X,DU Y,DENG Y M,et al. Groundwater discharge and lake water quality evolution[J]. Bulletin of Geological Science and Technology,2022,41(1):1-10. (in Chinese with English abstract [2] MAROUSEK J,GAVUROVA B. Recovering phosphorous from biogas fermentation residues indicates promising economic results[J]. Chemosphere,2022,291:133008. [3] LI X S,WANG Y M,HU Y J,et al. Numerical investigation on stratum and surface deformation in underground phosphorite mining under different mining methods[J]. Frontiers in Earth Science,2022,10:831856. [4] XUE Q,HE X Y,SACHS S B ,et al. The current phosphate recycling situation in China and Germany:A comparative review[J]. Frontiers of Agricultural Science and Engineering,2019,6(4):403-418. [5] TARTAKOVSKY D,STERN E,BRODAY D M. Indirect estimation of emission factors for phosphate surface mining using air dispersion modeling[J]. Science of the Total Environment,2016, 556:179-188. [6] ELSER J,BENNETT E. A broken biogeochemical cycle[J]. Nature,2011, 478:29-31. [7] SMITH V H,TILMAN G D,NEKOLA J C. Eutrophication:Impacts of excess nutrient inputs on freshwater,marine,and terrestrial ecosystems[J]. Environment Pollution,1999,100(1/3):179-196. [8] LI G H,VAN ITTERSUM M K,LEFFELAAR P A,et al. A multi-level analysis of China's phosphorus flows to identify options for improved management in agriculture[J]. Agricultural Systems,2016,144:87-100. doi: 10.1016/j.agsy.2016.01.006 [9] GILBERT N. Environment:The disappearing nutrient[J]. Nature , 2009, 461: 716-718. [10] SCHOLZ R W ,ULRICH A E,EILITTA M,et al. Sustainable use of phosphorus:A finite resource[J]. Science of the Total Environment,2013, 461:799-803. [11] CORDELL D,ROSEMARIN A,SCHRODER J J,et al. Towards global phosphorus security:A systems framework for phosphorus recovery and reuse options[J]. Chemosphere,2011,84(6):747-758. doi: 10.1016/j.chemosphere.2011.02.032 [12] HAO X D,WANG C C,VAN LOOSDRECHT M C M,et al. Looking beyond struvite for P-recovery[J]. Environmental Science & Technology,2013, 47 (10):4965-4966. [13] SASABUCHI I T M,KRIEGER K S,NUNES R S,et al. Sustainability in phosphorus use:A bibliographic review focusing on the current situation in the state of so paulo,Brazil[J]. Quimica Nova,2023,46(2):185-198. [14] MAROUSEK J,KOLAR L,STRUNECKY O,et al. Modified biochars present an economic challenge to phosphate management in wastewater treatment plants[J]. Journal of Cleaner Production,2020,272(8):123015. [15] GUTIERREZ F,KINNEY K A,KATZ L E. Phosphorus speciation in municipal wastewater solids and implications for phosphorus recovery[J]. Environmental Engineering Science,2020,37(5):316-327. doi: 10.1089/ees.2019.0360 [16] HONG S K D,WINKLER M K H,WANG Z W,et al. Integration of EBPR with mainstream anammox process to treat real municipal wastewater:Process performance and microbiology[J]. Water Research,2023,233:119758. [17] Kim K W,Kim Y J,Kim I T,et al. Electrochemical conversion characteristics of ammonia to nitrogen[J]. Water Research ,2006, 40(7):1431-1441. [18] JEONG B Y,SONG S H,BAEK K W,et al. Preparation and properties of heterogeneous cation exchange membrane for recovery of ammonium ion from waste water[J]. Polymer-Korea,2006,30(6):486-491. [19] MASSEY M S,DAVIS J G,SHEFFIELD R E,et al. In struvite production from dairy wastewater and its potential as a fertilizer for organic production in calcareous soils[C]//Anon. International Symposium on Air Quality and Waste Management for Agriculture. [S. l. ]:[S. n. ],2007. [20] MASINDI V,FOSSO-KANKEU E,MAMAKOA E,et al. Emerging remediation potentiality of struvite developed from municipal wastewater for the treatment of acid mine drainage[J]. Environmental Research,2022,210(15):112944. [21] STAVKOVA J,MAROUSEK J. Novel sorbent shows promising financial results on P recovery from sludge water[J]. Chemosphere,2021,276(8):130097. [22] SUN H J,MOHAMMED A N,LIU Y. Phosphorus recovery from source-diverted blackwater through struvite precipitation[J]. Science of the Total Environment ,2020, 743:140747. [23] MAYER B K,BAKER L A,BOYER T H,et al. Total value of phosphorus recovery[J]. Environmental Science & Technology,2016,50(13):6606-6620. [24] RAHMAN M M,SALLEH M A M,RASHID U,et al. Production of slow release crystal fertilizer from wastewaters through struvite crystallization :A review[J]. Arabian Journal of Chemistry,2014,7 (1):139-155. [25] RAHAMAN M S,MAVINIC D S. Recovering nutrients from wastewater treatment plants through struvite crystallization:CFD modelling of the hydrodynamics of UBC MAP fluidized-bed crystallizer[J]. Water Science and Technology,2009,59(10):1887-1892. doi: 10.2166/wst.2009.214 [26] TALBOYS P J,HEPPEL J,ROOSE T,HEALEY J R,et al. Struvite:A slow-release fertiliser for sustainable phosphorus management[J]. Plant and Soil ,2016, 401 (1/2):109-123. [27] RAHMAN M M,LIU Y,KWAG J H,et al. Recovery of struvite from animal wastewater and its nutrient leaching loss in soil[J]. Journal of Hazardous Materials,2011,186(2/3):2026-2030. [28] MUNCH E V,BARR K. Controlled struvite crystallisation for removing phosphorus from anaerobic digester sidestreams[J]. Water Research,2001,35(1):151-159. doi: 10.1016/S0043-1354(00)00236-0 [29] EL DIWANI G,EL RAFIE S,EL IBIARI N N,et al. Recovery of ammonia nitrogen from industrial wastewater treatment as struvite slow releasing fertilizer[J]. Desalination , 2007, 214 (1/3):200-214. [30] WU Q Z,BISHOP P L. Enhancing struvite crystallization from anaerobic supernatant[J]. Journal of Environmental Engineering and Science,2004,3(1):21-29. doi: 10.1139/s03-050 [31] KIM D,RYU H D,KIM M S,et al. Enhancing struvite precipitation potential for ammonia nitrogen removal in municipal landfill leachate[J]. Journal of Hazardous Materials,2007,146(1/2):81-85. [32] YANG H,WANG P L,CHEN A Q,et al. Prediction of phosphorus concentrations in shallow groundwater in intensive agricultural regions based on machine learning[J]. Chemosphere,2023,313(10):137126. [33] WARRACK J,KANG M,VON SPERBER C. Groundwater phosphorus concentrations:Global trends and links with agricultural and oil and gas activities[J]. Environmental Research Letters ,2022, 17(1):11.014014. [34] YU L,ROZEMEIJER J,VAN BREUKELEN B M,et al. Groundwater impacts on surface water quality and nutrient loads in lowland polder catchments:Monitoring the greater Amsterdam area[J]. Hydrology and Earth System Sciences,2018,22(1):487-508. [35] ZHOU J,DU Y,DENG Y M,et al. Source identification of groundwater phosphorus under different geological settings in the central Yangtze River Basin[J]. Journal of Hydrology,2022,612:13. 128169. [36] 冷智超,杜尧,陶艳秋,等,长江中游沿岸地下水中铵氮与磷的共存规律及其控制因素[J],地质科技通报,2022,41(1):300-308.LENG Z C,DU Y,TAO Y Q,et al. Coexistence of ammonium nitrogen and phosphorus in groundwater along the middle reaches of Yangtze River and its controlling factors[J],Bulletin of Geological Science and Technology,2022,41(1):300-308. (in Chinese with English abstract [37] NONGQWENGA N,MUCHAONYERWA P,HUGHES J,et al. Possible use of struvite as an alternative phosphate fertilizer[J]. Journal of Soil Science and Plant Nutrition ,2017, 17(3):581-593. [38] YAN H,SHIH K. Effects of calcium and ferric ions on struvite precipitation:A new assessment based on quantitative X-ray diffraction analysis[J]. Water Research,2016,95:310-318. doi: 10.1016/j.watres.2016.03.032 [39] ZHOU Z,HU D,REN W,et al. Effect of humic substances on phosphorus removal by struvite precipitation[J]. Chemosphere,2015,141:94-99. doi: 10.1016/j.chemosphere.2015.06.089 [40] WEI Q Q,CHEN J Y,ZHANG Q,et al. Insight into the effect of phosphate on ferrihydrite colloid-mediated transport of tetracycline in saturated porous media[J]. Environmental Science and Pollution Research ,2022, 29(53):80693-80704. [41] LI B,BOIARKINA I,YOUNG B,et al. Quantification and mitigation of the negative impact of calcium on struvite purity[J]. Advanced Powder Technology,2016,27(6):2354-2362. [42] TANSEL B,LUNN G,MONJE O. Struvite formation and decomposition characteristics for ammonia and phosphorus recovery:A review of magnesium-ammonia-phosphate interactions[J]. Chemosphere,2018,194:504-514. doi: 10.1016/j.chemosphere.2017.12.004 [43] MUSTAPHA S,TIJANI J O,NDAMITSO M M,et al. Facile synthesis and characterization of TiO2 nanoparticles:X-ray peak profile analysis using Williamson-Hall and Debye-Scherrer methods[J]. International Nano Letters ,2021, 11(3):241-261. [44] SONG Y H,YUAN P,ZHENG B H,et al. Nutrients removal and recovery by crystallization of magnesium ammonium phosphate from synthetic swine wastewater[J]. Chemosphere ,2007, 69(2):319-324. [45] ROUFF A A. The use of TG/DSC-FT-IR to assess the effect of Cr sorption on struvite stability and composition[J]. Journal of Thermal Analysis and Calorimetry ,2012, 110(3):1217-1223. [46] LEI Y,SONG B N,VAN DER WEIJDEN R D,et al. Electrochemical induced calcium phosphate precipitation:Importance of local pH[J]. Environmental Science & Technology,2017,51(19):11156-11164. [47] HUANG H M,XU C L,ZHANG W. Removal of nutrients from piggery wastewater using struvite precipitation and pyrogenation technology[J]. Bioresource Technology,2011,102(3):2523-2528. [48] SUNDA W,HUNTSMAN S. Effect of pH,light,and temperature on Fe-EDTA chelation and Fe hydrolysis in seawater[J]. Marine Chemistry,2003,84(1/2):35-47. [49] MBAMBA C K,TAIT S,FLORES-ALSINA X,et al. A systematic study of multiple minerals precipitation modelling in wastewater treatment[J]. Water Research,2015,85:359-370. doi: 10.1016/j.watres.2015.08.041 [50] LIZARRALDE I,FERNANDEZ-AREVALO T,BROUCKAERT C,et al. A new general methodology for incorporating physico-chemical transformations into multi-phase wastewater treatment process models[J]. Water Research,2015,74:239-256. doi: 10.1016/j.watres.2015.01.031 [51] PERWITASARI D S,MURYANTO S,SCHMAHL W W J,et al. A kinetic and structural analysis of the effects of Ca- and Fe ions on struvite crystal growth[J]. Solid State Sciences ,2022,134:107062. [52] ZHANG B,TIAN S Y,WU D L. Phosphorus harvesting from fresh human urine:A strategy of precisely recovering high-purity calcium phosphate and insights into the precipitation conversion mechanism[J]. Water Research,2022,227(11):119325. [53] JIANG L,ZHU J,WANG H,et al. Sorption of humic acid on Fe oxides,bacteria,and Fe oxide-bacteria composites[J]. Journal of Soils and Sediments,2014,14(8):1378-1384. [54] BOLEA E,GORRIZ M P,BOUBY M,et al. Multielement characterization of metal-humic substances complexation by size exclusion chromatography,asymmetrical flow field-flow fractionation,ultrafiltration and inductively coupled plasma-mass spectrometry detection:A comparative approach[J]. Journal of Chromatography A, 2006,1129(2):236-246. [55] BANIK N L,BUDA R A,BURGER S,et al. Speciation and interactions of plutonium with humic substances and kaolinite in aquifer systems[J]. Journal of Alloys and Compounds,2007,444:522-525. [56] ZUO H Y,HUANG L Q,CHU R K,et al. Reduction of structural Fe(III) in nontronite by humic substances in the absence and presence of Shewanella putrefaciens and accompanying secondary mineralization[J]. American Mineralogist,2021,106(12):1957-1970. [57] LIU Y T,HESTERBERG D. Phosphate bonding on noncrystalline Al/Fe-hydroxide coprecipitates[J]. Environmental Science & Technology,2011,45(15):6283-6289. [58] ARSLANOGLU H. Adsorption of micronutrient metal ion onto struvite to prepare slow release multielement fertilizer:Copper(II) doped-struvite[J]. Chemosphere,2019,217:393-401. [59] TANG Z J,HONG S K,XIAO W Z,et al. Characteristics of iron corrosion scales established under blending of ground,surface,and saline waters and their impacts on iron release in the pipe distribution system[J]. Corrosion Science,2006,48(2):322-342. [60] LEVSHINA S. An assessment of metal-humic complexes in river waters of the Upper Amur Basin,Russia[J]. Environ. Monit. Assessment,2018,190(1):13. [61] WANG J,BURKEN J G,ZHANG X,et al. Engineered struvite precipitation:Impacts of component-ion molar ratios and pH[J]. Journal of Environmental Engineering ,2005, 131(10):1433-1440. [62] PAN Z Q,HU M P,SHEN H,et al. Quantifying groundwater phosphorus flux to rivers in a typical agricultural watershed in eastern China[J]. Environmental Science and Pollution Research,2023,30(8):19873-19889. [63] LI Y P,DU Y,DENG Y M,et al. Predicting the spatial distribution of phosphorus concentration in Quaternary sedimentary aquifers using simple field parameters[J]. Applied Geochemistry,2022,142:105349. -





 下载:
下载: