Factors influencing the dissolution rate of residual DNAPL in unconnected pores based on PIV technology
-
摘要:
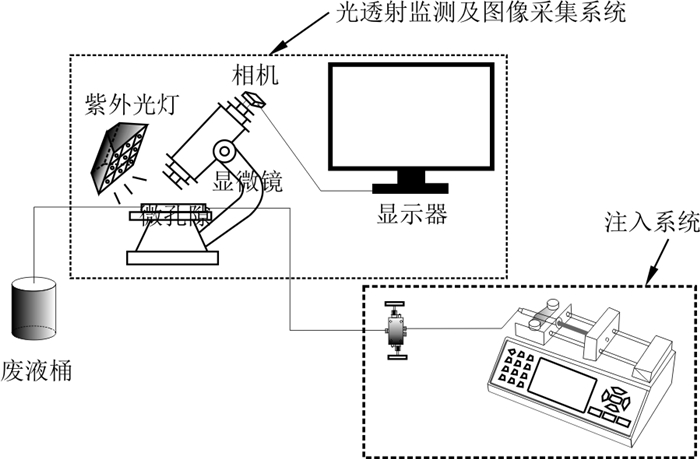
密度大于水的重非水相液体(dense non-aqueous phase liquids, 简称DNAPL)泄露进入地下环境成为长期的污染源。前人基于土柱、砂箱实验及数值模拟等手段研究了冲洗液流速、助溶剂浓度及介质性质对DNAPL清除效率的影响, 但是孔隙流速如何影响孔隙尺度残余DNAPL的溶解速率还不清楚。在微孔隙模型中注入乙醇冲洗液模拟孔隙中残余PCE的清除过程, 利用微尺度粒子图像测速技术(PIV)获取孔隙通道中的水相流速场分布, 分析不同孔隙结构中的残余PCE溶解清除速率的影响因素。实验结果表明: 影响不连通孔隙残余PCE溶解速率
R 的因素不仅仅是水相流速, 还有孔隙断面通量q 、孔隙开口方向和孔隙通道水相流速方向的夹角α 以及通量梯度I 等; 基于实验数据拟合得出溶解速率与影响因素之间的定量关系为R =3 876.79q (-0.016α+2.28/I )2;q 越大, 不连通孔隙附近的孔隙通道水相更新速率就越快;α 越大(α >90°), 有更多的冲洗液进入不连通孔隙内, 从而增加残余PCE溶解速率;I 越大, 垂直进入不连通孔隙内部的水相通量的分量衰减越快, 界面附近的流速就会越小, 残余PCE的溶解速率减小。基于微孔隙PIV技术定量揭示了孔隙流速及介质孔隙结构等多因素共同影响孔隙中DNAPL的溶解速率, 为深入理解孔隙中残余DNAPL的溶解机理、定量评估实际场地条件下残余DNAPL清除效率提供新的手段。Abstract:Objective The leakage of dense nonaqueous phase liquids (DNAPL) with a density greater than that of water into the underground environment becomes a long-term pollution source. Previous researchers have studied the effects of flushing fluid flow rate, cosolvent concentration, and media properties on DNAPL removal efficiency through methods such as column experiments, sandboxes, and numerical simulations.
Methods However, the effect of pore-scale flow rate on the dissolution rate of residual DNAPL in the pores remains unclear. In this study, ethanol flushing solution was injected into a micropore model to simulate the removal process of residual PCE from pores. Microscale particle image velocimetry (PIV) was used to obtain the distribution of the water phase velocity field in the pore channel, and the factors affecting the dissolution and removal rate of residual PCE in different pore structures were analysed.
Results The experimental results indicate that the dissolution rate (
R ) of residual PCE in unconnected pores is influenced by several factors, including the water phase flow rate, the cross-sectional flux (q ) within the pore, the angle (α ) between the direction of pore opening and the direction of water phase flow, and the flux gradient (I ). Based on fitting the experimental data, the quantitative relationship between dissolution rate and influencing factors is obtained as follows:R =3 876.79q (-0.016a+2.28/I )2. A larger value ofq corresponds to a higher renewal rate of the water phase in pore channels near unconnected pores, leading to an increased dissolution rate of residual PCE. Whenα is larger (α >90°), more flushing fluid enters the unconnected pores, further enhancing the dissolution rate of residual PCE. On the other hand, a larger value ofI leads to a faster attenuation of the vertical component of the water phase flux entering the unconnected pores. This results in lower flow velocities near the interface, causing a decrease in the dissolution rate of residual PCE.Conclusion Based on micropore PIV technology, multiple factors, such as pore flow rate and medium pore structure, have been quantitatively revealed to jointly affect the dissolution rate of DNAPL in pores. This finding offers a novel approach to enhance our the understanding of the dissolution mechanism of residual DNAPL in pores and to quantitatively assess the removal efficiency of residual DNAPL under real-site conditions.
-
Key words:
- pore velocity /
- micropore-model /
- dissolution rate /
- unconnected pore
-
表 1 微孔隙模型参数
Table 1. Micropore model parameters
参数 数值 长度/mm 20.089 宽度/mm 15.866 厚度/mm 0.931 孔隙度/% 63 表 2 不连通孔隙残余PCE溶解速率的影响因素
Table 2. Influencing factors of the dissolution rate of residual PCE in unconnected pores
点位 孔隙断面通量/(10-7m2·s-1) 角度/(°) 通量梯度/(10-4m·s-1) 溶解速率/(μm2·min-1) A1 1.32 130.4 0.58 9 425.9 A2 1.11 105.4 1.48 4 102.5 A3 0.92 133 0.94 5 477.1 A4 1.41 72.7 1.74 2 909.0 A5 1.26 142.1 2.22 5 728.6 A6 1.07 90.7 1.43 3 176.2 A7 0.43 142.1 0.75 2 421.0 -
[1] Essaid H I, Bekins B A, Cozzarelli I M. Organic contaminant transport and fate in the subsurface: Evolution of knowledge and understanding[J]. Water Resources Research, 2015, 51(7): 4861-4902. doi: 10.1002/2015WR017121 [2] Agaoglu B, Copty N K, Scheytt T, et al. Interphase mass transfer between fluids in subsurface formations: A review[J]. Advances in Water Resources, 2015, 79: 162-194. doi: 10.1016/j.advwatres.2015.02.009 [3] 甘义群, 于凯, 周爱国, 等. 基于GasBench-IRMS的挥发性氯代烃碳氯同位素指纹特征分析[J]. 地质科技情报, 2013, 32(6): 110-115. https://www.cnki.com.cn/Article/CJFDTOTAL-DZKQ201306018.htmGan Y Q, Yu K, Zhou A G, et al. Isotopic fingerprint analysis of carbon and chlorine of volatile chlorinated hydrocarbons based on GasBench-IRMS[J]. Geological Science and Technology Information, 2013, 32(6): 110-115(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-DZKQ201306018.htm [4] 宋美钰, 施小清, 康学远, 等. DNAPL场地污染通量升尺度预测的敏感性分析[J]. 地质科技通报, 2023, 42(2): 327-335. doi: 10.19509/j.cnki.dzkq.tb20220262Song M Y, Shi X Q, Kang X Y, et al. Sensitivity analysis of upscaling prediction of the mass flux at DNAPL contaminated sites[J]. Bulletin of Geological Science and Technology, 2023, 42(2): 327-335(in Chinese with English abstract). doi: 10.19509/j.cnki.dzkq.tb20220262 [5] 蒲生彦, 唐菁, 侯国庆, 等. 缓释型化学氧化剂在地下水DNAPLs污染修复中的应用研究进展[J]. 环境化学, 2020, 39(3): 791-799. https://www.cnki.com.cn/Article/CJFDTOTAL-HJHX202003023.htmPu S Y, Tang J, Hou G Q, et al. Research progress of application of slow-release chemical oxidant in remediation of DNAPLs pollution in groundwater[J]. Environmental Chemistry, 2020, 39(3): 791-799(in Chinese with English abstract). https://www.cnki.com.cn/Article/CJFDTOTAL-HJHX202003023.htm [6] Hunt J R, Sitar N, Udell K S. Nonaqueous phase liquid transport and cleanup: 1. Analysis of mechanisms[J]. Water Resources Research, 1988, 24(8): 1247-1258. doi: 10.1029/WR024i008p01247 [7] Karaoglu A G, Copty N K, Akyol N H, et al. Experiments and sensitivity coefficients analysis for multiphase flow model calibration of enhanced DNAPL dissolution[J]. Journal of Contaminant Hydrology, 2019, 225: 103515. doi: 10.1016/j.jconhyd.2019.103515 [8] 周媛, 杨盼瑞, 郭会荣, 等. 注入丁醇调节重非水液相密度的微空隙试验模拟[J]. 地质科技通报, 2022, 41(1): 223-230. doi: 10.19509/j.cnki.dzkq.2022.0016Zhou Y, Yang P R, Guo H R, et al. Injecting n-BuOH to achieve density conversion of dense non-aqueous phase liquid: Pore-scale experimental simulation[J]. Bulletin of Geological Science and Technology, 2022, 41(1): 223-230(in Chinese with English abstract). doi: 10.19509/j.cnki.dzkq.2022.0016 [9] 付玉丰. 表面活性剂及其复配体系对DNAPL污染含水层的增溶增流修复研究[D]. 长春: 吉林大学, 2020.Fu Y F. Study on solubilization and flow-increasing remediation of DNAPL contaminated aquifer by surfactant and its complex system[D]. Changchun: Jilin University, 2020(in Chinese with English abstract). [10] Luciano A, Mancini G, Torretta V, et al. An empirical model for the evaluation of the dissolution rate from a DNAPL-contaminated area[J]. Environmental Science and Pollution Research, 2018, 25(34): 33992-34004. doi: 10.1007/s11356-018-3193-6 [11] Sarikurt D A, Gokdemir C, Copty N K. Sherwood correlation for dissolution of pooled NAPL in porous media[J]. Journal of Contaminant Hydrology, 2017, 206: 67-74. doi: 10.1016/j.jconhyd.2017.10.001 [12] Tatti F, Papini M P, Sappa G, et al. Contaminant back-diffusion from low-permeability layers as affected by groundwater velocity: A laboratory investigation by box model and image analysis[J]. Science of the Total Environment, 2018, 622: 164-171. [13] Karaoglu A G, Copty N K, Akyol N H, et al. Experiments and sensitivity coefficients analysis for multiphase flow model calibration of enhanced DNAPL dissolution[J]. Journal of Contaminant Hydrology, 2019, 225: 103515. doi: 10.1016/j.jconhyd.2019.103515 [14] Yaksi K, Demiray Z, Copty N K. Impact of cosolvents on the interphase mass transfer of NAPLs in porous media[J]. Water Resources Research, 2021, 57(8): e2020WR029326. [15] Alazaiza M Y D, Copty N K, Abunada Z. Experimental investigation of cosolvent flushing of DNAPL in double-porosity soil using light transmission visualization[J]. Journal of Hydrology, 2020, 584: 124659. [16] Hu Y, Patmonoaji A, Xu H, et al. Pore-scale investigation on nonaqueous phase liquid dissolution and mass transfer in 2D and 3D porous media[J]. International Journal of Heat and Mass Transfer, 2021, 169: 120901. doi: 10.1016/j.ijheatmasstransfer.2021.120901 [17] Li M, Zhai Y, Wan L. Measurement of NAPL: Water interfacial areas and mass transfer rates in two-dimensional flow cell[J]. Water Science and Technology, 2016, 74(9): 2145-2151. doi: 10.2166/wst.2016.397 [18] Santiago J G, Wereley S T, Meinhart C D, et al. A particle image velocimetry system for microfluidics[J]. Experiments in fluids, 1998, 25(4): 316-319. [19] Koutsiaris A G, Mathioulakis D S, Tsangaris S. Microscope PIV for velocity-field measurement of particle suspensions flowing inside glass capillaries[J]. Measurement Science and Technology, 1999, 10(11): 1037-1046. [20] Heshmati M, Piri M. Interfacial boundary conditions and residual trapping: A pore-scale investigation of the effects of wetting phase flow rate and viscosity using micro-particle image velocimetry[J]. Fuel, 2018, 224: 560-578. [21] Roman S, Soulaine C, AlSaud M A, et al. Particle velocimetry analysis of immiscible two-phase flow in micromodels[J]. Advances in Water Resources, 2016, 95(S1): 199-211. [22] Sen D, Nobes D S, Mitra S K. Optical measurement of pore scale velocity field inside microporous media[J]. Microfluidics and Nanofluidics, 2012, 12(1/4): 189-200. [23] Thielicke W, Stamhuis E. PIVlab: Towards user-friendly, affordable and accurate digital particle image velocimetry in MATLAB[J]. Journal of Open Research Software, 2014, 2: e30. [24] Scarano F, Riethmuller M L. Iterative multigrid approach in PIV image processing with discrete window offset[J]. Experiments in Fluids, 1999, 26(6): 513-523. [25] Willert C E, Gharib M. Digital particle image velocimetry[J]. Experiments in Fluids, 1991, 10(4): 181-193. [26] Blois G, Barros J M, Christensen K T. A microscopic particle image velocimetry method for studying the dynamics of immiscible liquid-liquid interactions in a porous micromodel[J]. Microfluidics and Nanofluidics, 2015, 18(5/6): 1391-1406. [27] Cho J, Annable M D, Rao P S C. Measured mass transfer coefficients in porous media using specific interfacial area[J]. Environmental Science & Technology, 2005, 39(20): 7883-7888. [28] Luciano A, Mancini G, Torretta V, et al. An empirical model for the evaluation of the dissolution rate from a DNAPL-contaminated area[J]. Environmental Science and Pollution Research, 2018, 25(34): 33992-34004. -





 下载:
下载: