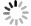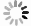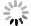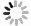-
摘要:
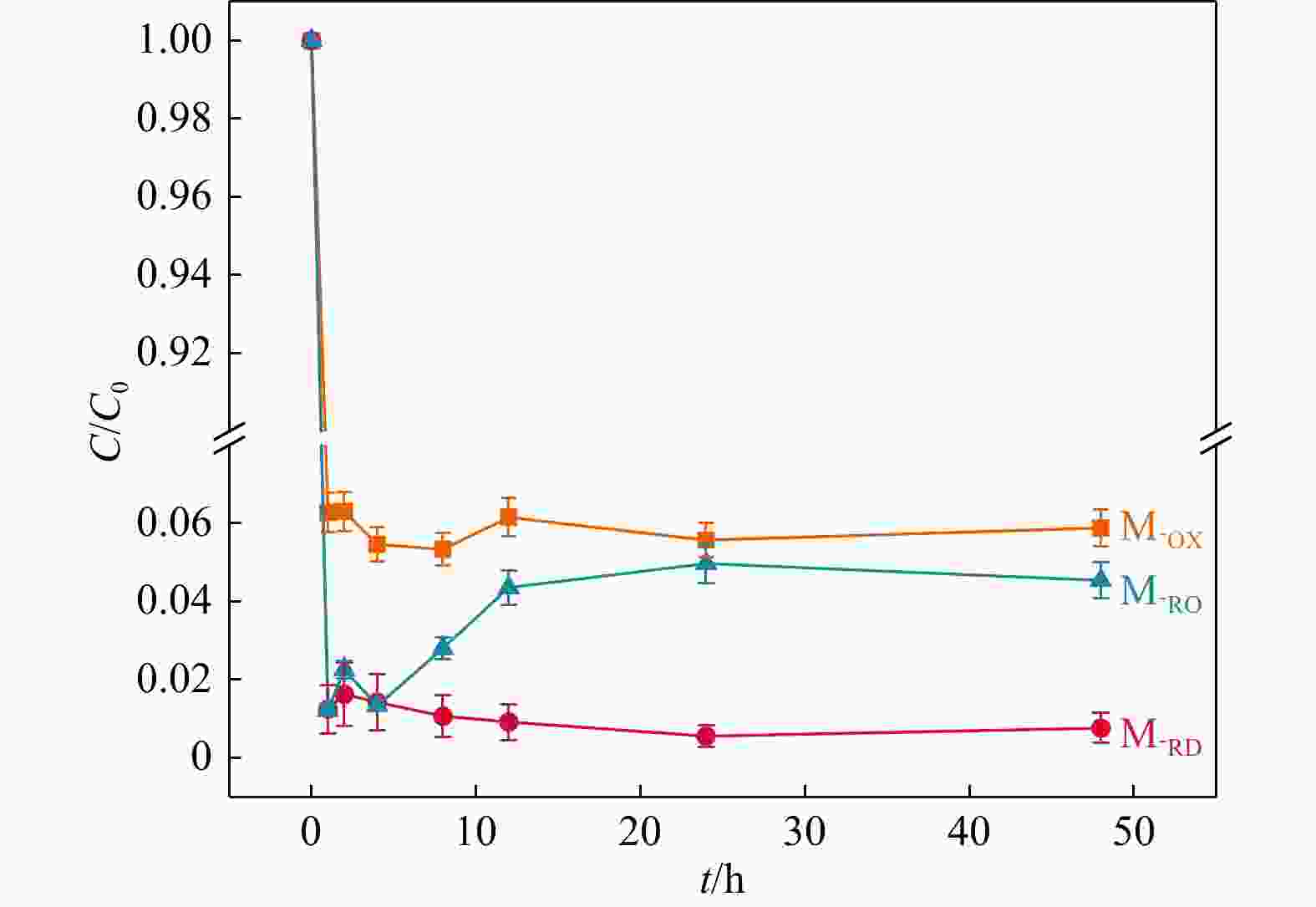
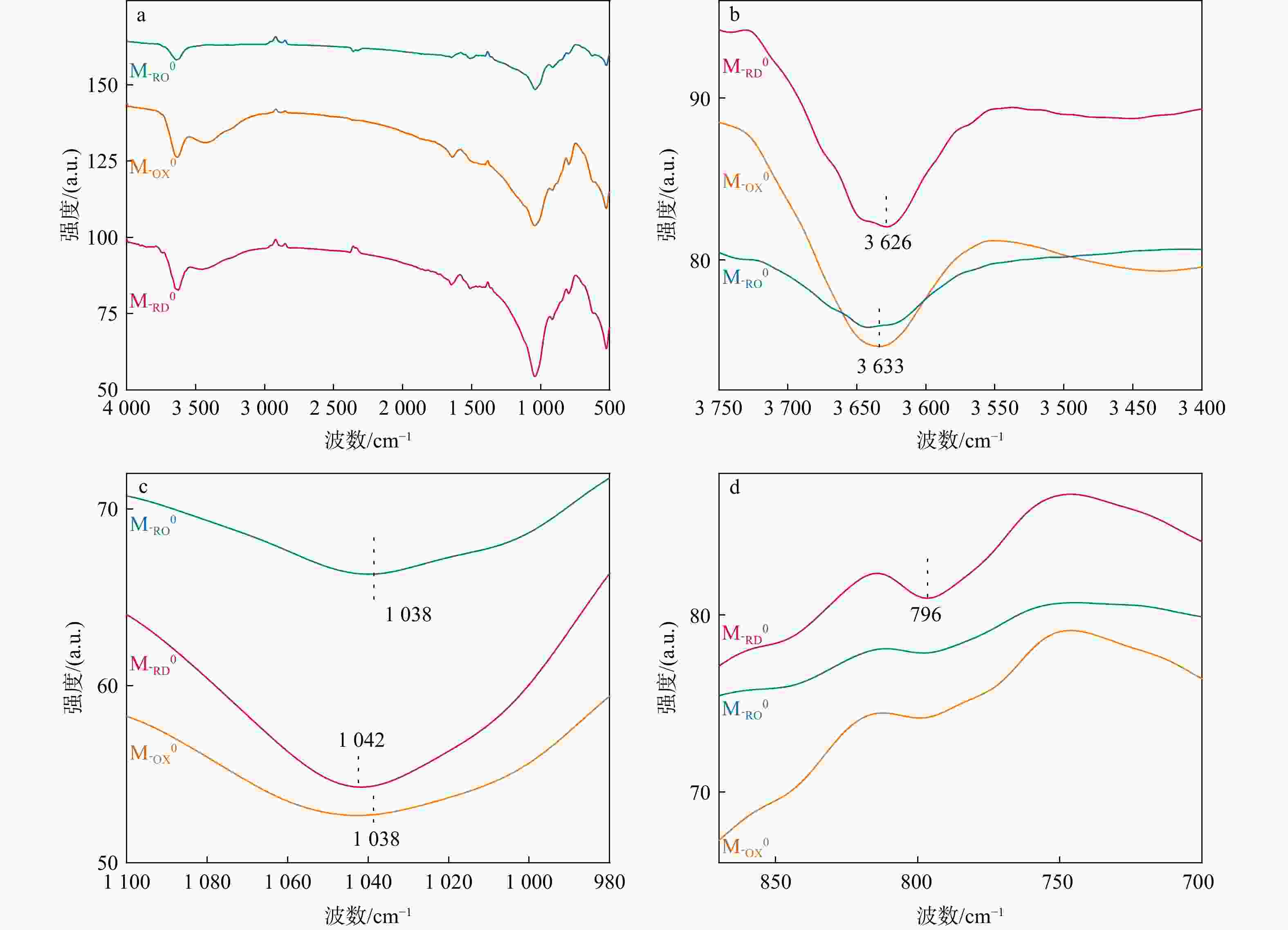
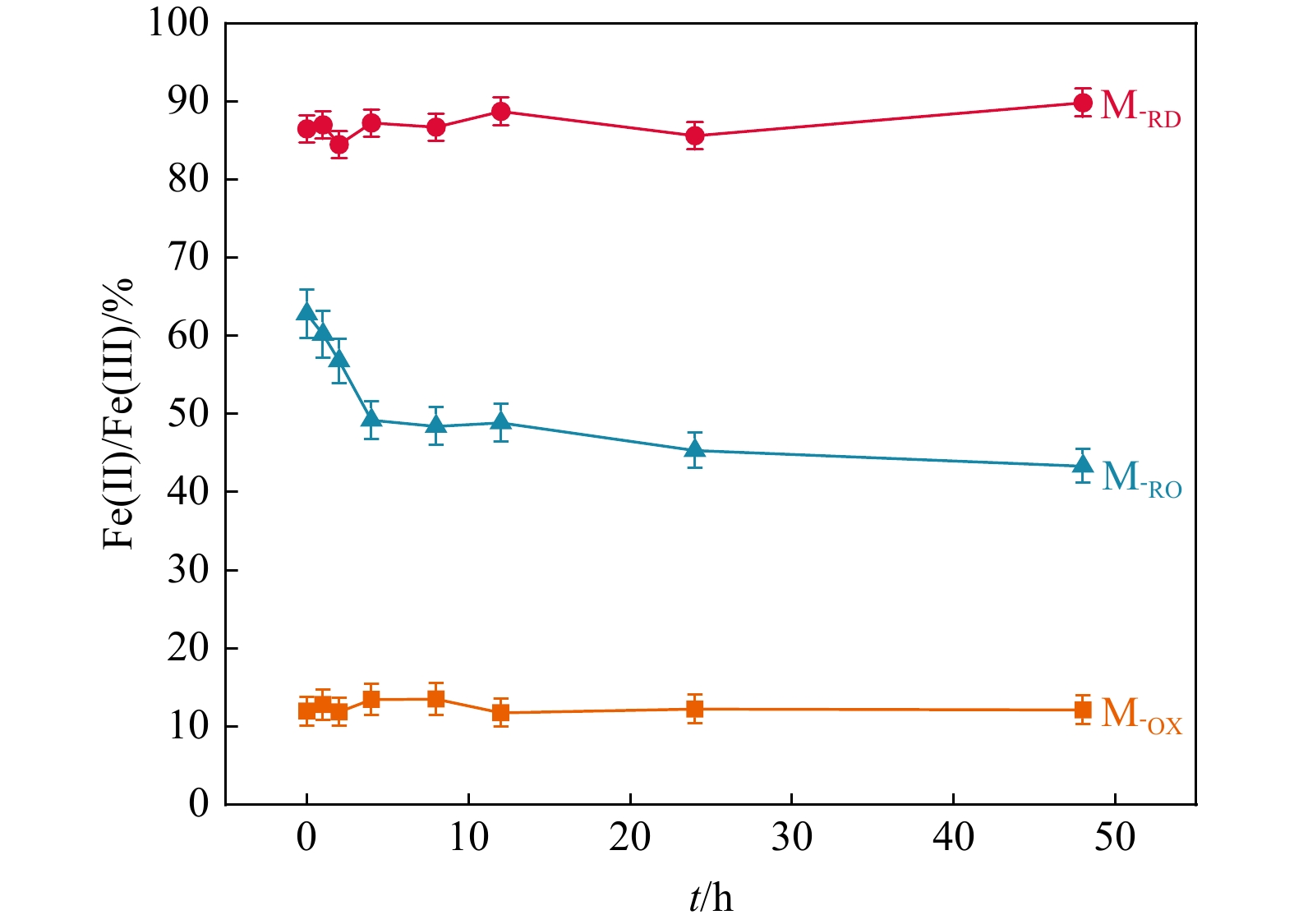
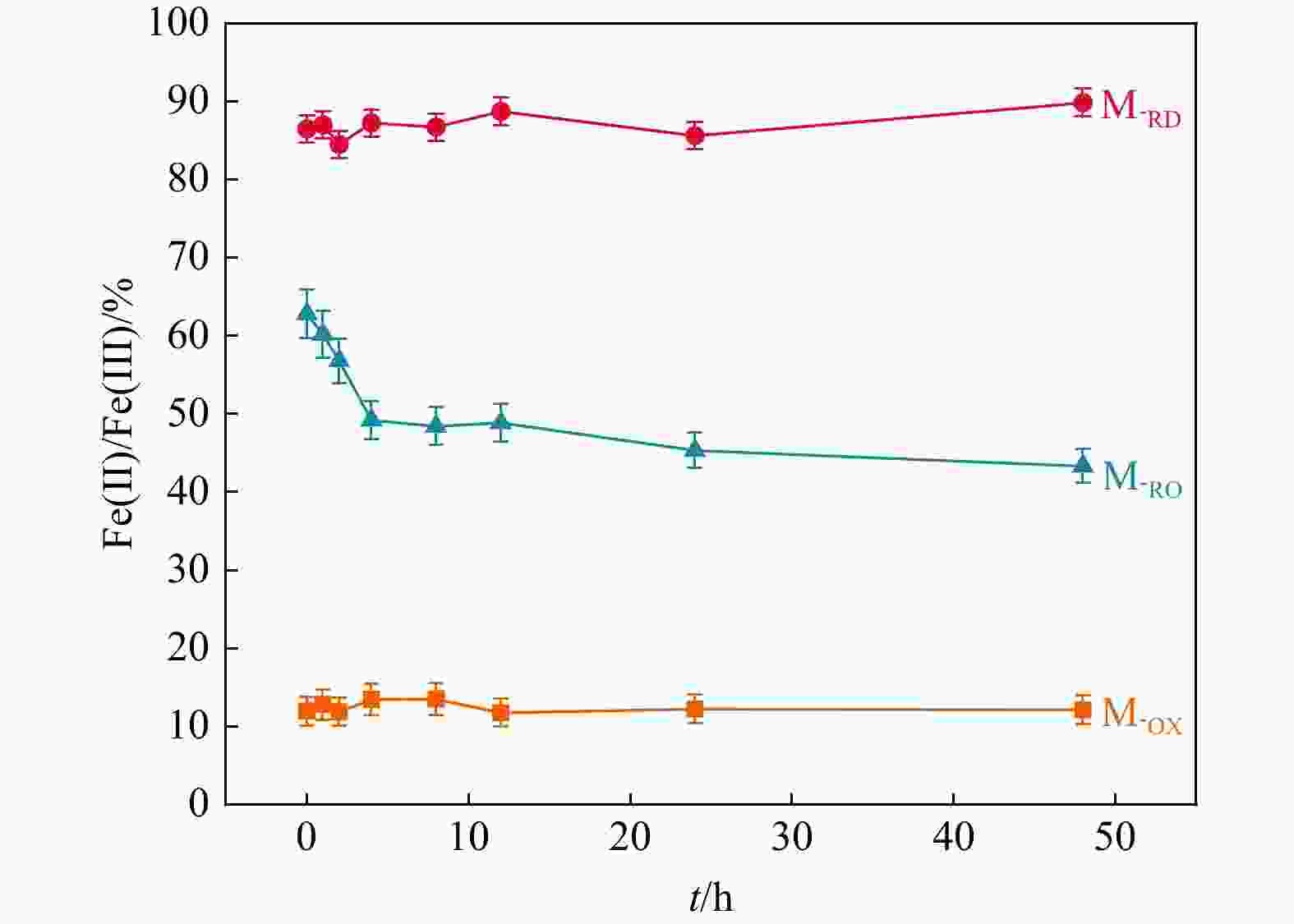
地下氧化还原环境变化影响重金属在土壤中的吸附解吸,其影响机制尚不明朗。制备了不同氧化还原环境的蒙脱石,采用静态吸附实验及表征手段探究了不同氧化还原环境对镉吸附的影响。结果显示,还原态蒙脱石M-RD和氧化态蒙脱石M-OX氧化还原性变化不大,还原再氧化态蒙脱石M-RO中发生的氧化还原反应最强烈,还原性逐渐下降。M-RD吸附效果比M-OX更好,尽管M-RD再次氧化后,部分被吸附的Cd会被释放出来,但效果还是高于M-OX。M-RD再次接触氧气时,与氧气发生氧化反应,产出大量羟基自由基,而M-OX和M-RD几乎不产生羟基自由基。M-RD再次氧化后,Fe(Ⅱ)-Fe(Ⅱ)-Fe(Ⅱ)-OH重排-OH弯曲振动特征吸收峰和Si-O四面体结构的特征吸收峰产生变动,表明蒙脱石发生了结构摄动,判断是其中的Fe(Ⅱ)失去电子被氧化成Fe(Ⅲ),导致了蒙脱石结构的变化,使得蒙脱石比表面积、孔容和平均孔径均增大了,氧化还原条件变化影响其吸附性能,削弱了蒙脱石对Cd的吸附,导致吸附的Cd被再次释放。本研究揭示了地下环境氧化还原条件影响Cd吸附解吸的机理,可为氧化还原环境不断变化的土壤污染修复治理提供理论指导。
Abstract:Objective Changes in subsurface redox environment can affect the adsorption and desorption of heavy metals in soils; however, the underlying mechanisms of these effects remain unclear.
Methods In this work, montmorillonite with different redox environments was prepared, and the effects of different redox environments on cadmium adsorption were assessed via static adsorption experiments and various characterization techniques.
Results The results revealed minimal changes in the redox properties of both reduced montmorillonite M-RD and oxidized montmorillonite M-OX. The strongest redox reaction was observed in reduced reoxidized montmorillonite M-RO, which showed a gradual decrease in the reducing properties. M-RD exhibited superior cadmium adsorption compared to M-OX; however, upon reoxidation, some adsorbed cadmium was released from M-RD, although the adsorption was still more effective than in M-OX. Re-exposure of M-RD to oxygen initiated an oxidation reaction, generating numerous hydroxyl radicals, a phenomenon not observed in M-OX or M-RD alone.
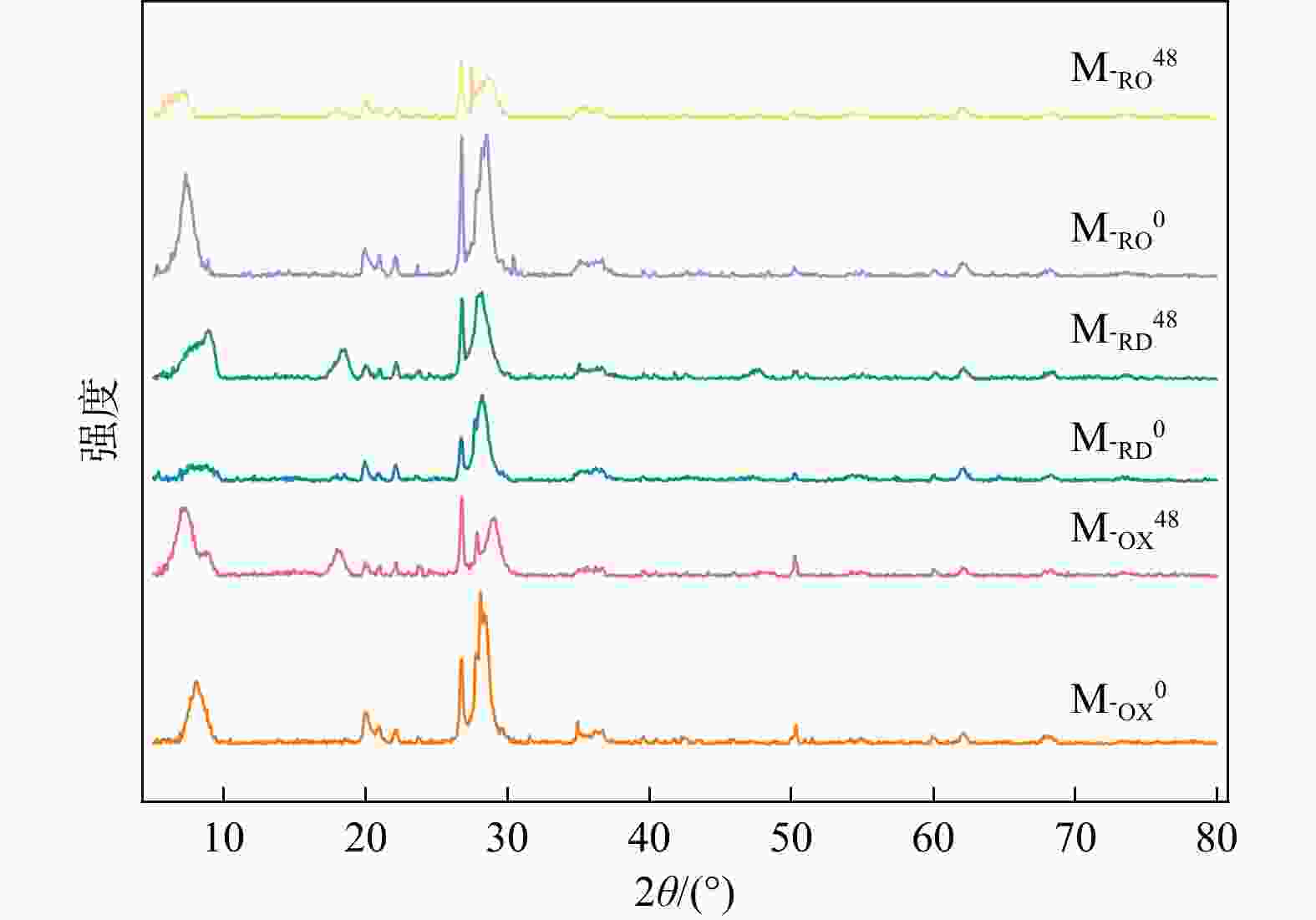
Conclusion Upon reoxidation of M-RD, changes occurred in the characteristic absorption peaks associated with Fe(Ⅱ)-Fe(Ⅱ)-Fe(Ⅱ)-Fe(Ⅱ)-Fe(Ⅱ)-OH rearrangement-OH bending vibrations and the Si-O tetrahedral structure, indicating structural uptake of montmorillonite. This suggests that Fe(Ⅱ) in the structure lost electrons, transforming into Fe(Ⅲ), thereby causing a structural change in montmorillonite. These changes led to increased specific surface area, pore volume, and average pore size of montmorillonite, ultimately affecting its adsorption capacity. The altered redox conditions weakened the adsorption of Cd, causing its release from montmorillonite. Uncovering the mechanisms of cadmium adsorption and desorption affected by redox conditions in subsurface environments can provide theoretical insights for the remediation and treatment of soil pollution in dynamic redox settings.
-
Key words:
- cadmium /
- redox /
- adsorption resolution /
- hydroxyl radicals.
-
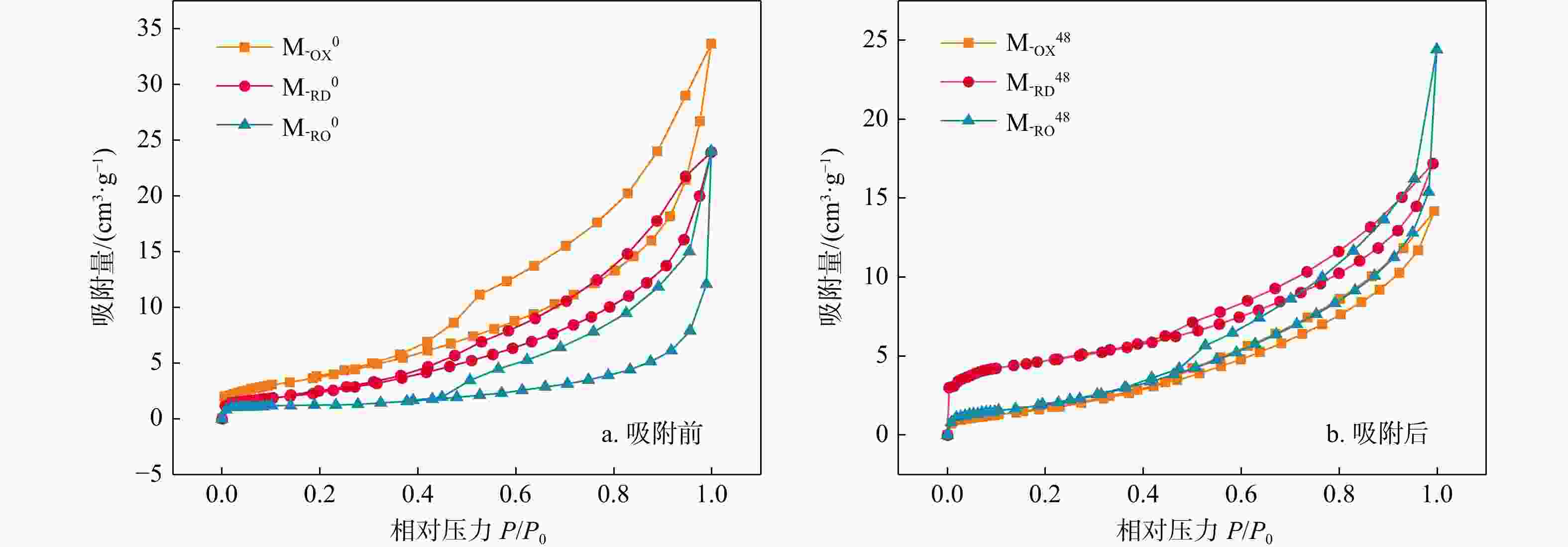
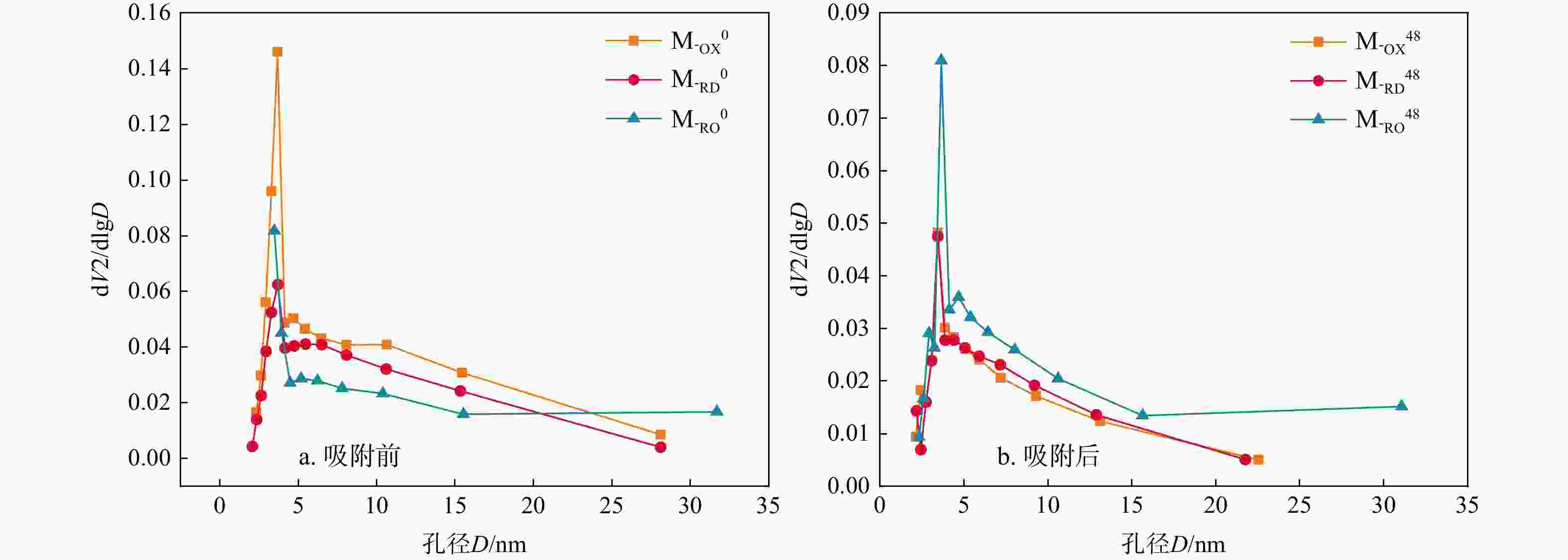
表 1 三种材料吸附前后的比表面积、孔容及平均孔径
Table 1. Specific surface area, pore volume and average pore size before and after adsorption of the three materials
编号 比表面积/(m2·g−1) 孔容/(cm3·g−1) 平均孔径/nm M-OX0 14.619 0.052 4.129 M-RD0 6.804 0.022 3.885 M-RO0 9.522 0.037 4.154 M-OX48 16.050 0.027 3.884 M-RD48 4.059 0.037 3.933 M-RO48 7.618 0.038 4.126 -
[1] LI Q,WANG C,DAI T,et al. Prediction of soil cadmium distribution across a typical area of Chengdu Plain,China[J]. Scientific Reports,2017,7(1):1-12. [2] TARAKINA N V,VERBERCK B. A portrait of cadmium[J]. Nature Chemistry,2016,9(1):96. [3] HU B,SHAO S,FU Z,et al. Identifying heavy metal pollution hot spots in soil-rice systems:A case study in south of Yangtze River Delta,China[J]. Science of the Total Environment,2019,658:614-625. doi: 10.1016/j.scitotenv.2018.12.150 [4] ZHANG X,ZHONG T,LIU L,et al. Impact of soil heavy metal pollution on food safety in China[J]. PloS One,2015,10(8):e0135182. doi: 10.1371/journal.pone.0135182 [5] QIN G,NIU Z,YU J,et al. Soil heavy metal pollution and food safety in China:Effects,sources and removing technology[J]. Chemosphere,2021,267:129205. doi: 10.1016/j.chemosphere.2020.129205 [6] HUANG,WANG L,WANG W,et al. Current status of agricultural soil pollution by heavy metals in China:A meta-analysis[J]. Science of the Total Environment,2019,651:3034-3042. doi: 10.1016/j.scitotenv.2018.10.185 [7] 刘剑峰,谷宁,张可慧. 土壤重金属空间分异及迁移研究进展与展望[J]. 地理与地理信息科学,2012,28(2):99-103.LIU J F,GU N,ZHANG K H. Progress and prospect of soil heavy metal spatial differentiation and migration[J]. Geography and Geo-Information Science,2012,28(2):99-103. (in Chinese with English abstract [8] 赵萌,姜永海,冯帆,等. 典型地球化学与水文地质特征对污染物自然衰减影响研究进展[J]. 地质科技通报,2023,42(3):250-261.ZHAO M,JIANG Y H,FENG F,et al. Resarch advances on the influence of typical geochemical and hydrogeological characteristics on the natural attenuation of pollutants[J]. Bulletin of Geological Science and Technology,2023,42(3):250-261. (in Chinese with English abstract [9] 尹元雪,赵雨溪,孙群群,等. Cr(Ⅲ)对锰氧化菌P. putida MnB1活性及功能的影响规律与机制[J]. 地质科技通报,2024,43(1):298-305.YIN Y X,ZHAO Y X,SUN Q Q,et al. Effect of Cr(Ⅲ) on the activity and function of Mn(Ⅱ)-oxidizing bacteria Pseudomonas putida MnB1[J]. Bulletin of Geological Science and Technology,2024,43(1):298-305. (in Chinese with English abstract [10] 巩宗强,李培军,台培东. 污染土壤的淋洗法修复研究进展[J]. 环境污染治理技术与设备,2002,3(7):45-50.GONG Z Q,LI P J,TAI P D. Advance ment of soil washing process for contaminated soil[J]. Techniques and Equipment for Environmental Pollution Control,2002,3(7):45-50. (in Chinese with English abstract [11] 刘慧. 巯基化蒙脱石的制备及其对镉的吸附/解吸机理研究 [D]. 成都:成都理工大学,2013.LIU H. Preparation of thiol-dodified montmorillonite and mechanism study of cadmium adsorption/desorption [D]. Chengdu:Chengdu University of Technology,2013. (in Chinese with English abstract [12] 曹春艳. 改性膨润土吸附处理 含六价铬废水的研究[J]. 化学工程师,2008(10):43-45. doi: 10.3969/j.issn.1002-1124.2008.10.016CAO C Y. Study on adsorption of charomium in wastewater by modified bentonite[J]. Chemical Engineer,2008(10):43-45. (in Chinese with English abstract doi: 10.3969/j.issn.1002-1124.2008.10.016 [13] 施和平,吴瑞凤,杨威. 蒙脱石的开发与应用[J]. 内蒙古石油化工,2004,30(2):32-34. doi: 10.3969/j.issn.1006-7981.2004.02.011SHI H P,WU R F,YANG W. Development and application of montmorillonite[J]. Inner Mongolia Petrochemicals,2004,30(2):32-34. (in Chinese with English abstract doi: 10.3969/j.issn.1006-7981.2004.02.011 [14] LIU X,YUAN S,TONG M,et al. Oxidation of trichloroethylene by the hydroxyl radicals produced from oxygenation of reduced nontronite[J]. Water Research,2017,113:72-79. doi: 10.1016/j.watres.2017.02.012 [15] GAN H,STUCKI J W,BAILEY G W. Reduction of structural iron in ferruginous smectite by free radicals[J]. Clays and Clay Minerals,1992,40(6):659-665. doi: 10.1346/CCMN.1992.0400605 [16] STUCKI J W,GOLDEN D,ROTH C B. Preparation and handling of dithionite-reduced smectite suspensions[J]. Clays and Clay Minerals,1984,32(3):191-197. doi: 10.1346/CCMN.1984.0320306 [17] JOO S H,FEITZ A J,SEDLAK D L,et al. Quantification of the oxidizing capacity of nanoparticulate zero-valent iron[J]. Environmental Science & Technology,2005,39(5):1263-1268. [18] KING D W,LOUNSBURY H A,MILLERO F J. Rates and mechanism of Fe (Ⅱ) oxidation at nanomolar total iron concentrations[J]. Environmental Science & Technology,1995,29(3):818-824. [19] PHAM A N,WAITE T D. Oxygenation of Fe (Ⅱ) in natural waters revisited:Kinetic modeling approaches,rate constant estimation and the importance of various reaction pathways[J]. Geochimica et Cosmochimica Acta,2008,72(15):3616-3630. doi: 10.1016/j.gca.2008.05.032 [20] MINELLA M,DE LAURENTIIS E,MAURINO V,et al. Dark production of hydroxyl radicals by aeration of anoxic lake water[J]. Science of the Total Environment,2015,527:322-327. [21] TONG M,YUAN S,MA S,et al. Production of abundant hydroxyl radicals from oxygenation of subsurface sediments[J]. Environmental Science & Technology,2016,50(1):214-221. [22] MOPPER K,ZHOU X. Hydroxyl radical photoproduction in the sea and its potential impact on marine processes[J]. Science,1990,250:661-664. doi: 10.1126/science.250.4981.661 [23] WANG X,DONG H,ZENG Q,et al. Reduced iron-containing clay minerals as antibacterial agents[J]. Environmental Science & Technology,2017,51(13):7639-7647. [24] HESTER E T,GOOSEFF M N. Moving beyond the banks:Hyporheic restoration is fundamental to restoring ecological services and functions of streams[J]. Environmental Science & Technology,2010,44(5):1521-1525. [25] BECK M,DELLWIG O,SCHNETGER B,et al. Cycling of trace metals (Mn,Fe,Mo,U,V,Cr) in deep pore waters of intertidal flat sediments[J]. Geochimica et Cosmochimica Acta,2008,72(12):2822-2840. doi: 10.1016/j.gca.2008.04.013 [26] KUMAR A R,RIYAZUDDIN P. Seasonal variation of redox species and redox potentials in shallow groundwater:A comparison of measured and calculated redox potentials[J]. Journal of Hydrology,2012,444:187-198. [27] ZHANG P,YUAN S,LIAO P. Mechanisms of hydroxyl radical production from abiotic oxidation of pyrite under acidic conditions[J]. Geochimica et Cosmochimica Acta,2016,172:444-457. [28] CHENG D,YUAN S,LIAO P,et al. Oxidizing impact induced by mackinawite (FeS) nanoparticles at oxic conditions due to production of hydroxyl radicals[J]. Environmental Science & Technology,2016,50(21):11646-11653. [29] ZHAO L,DONG H,KUKKADAPU R,et al. Biological oxidation of Fe (Ⅱ) in reduced nontronite coupled with nitrate reduction by Pseudogulbenkiania sp. Strain 2002[J]. Geochimica et Cosmochimica Acta,2013,119:231-247. doi: 10.1016/j.gca.2013.05.033 [30] ZHAO L,DONG H,KUKKADAPU R K,et al. Biological redox cycling of iron in nontronite and its potential application in nitrate removal[J]. Environmental Science & Technology,2015,49(9):5493-5501. [31] VANTELON D,MONTARGèS-PELLETIER E,MICHOT L,et al. Iron distribution in the octahedral sheet of dioctahedral smectites: An Fe K-edge X-ray absorption spectroscopy study[J]. Physics and Chemistry of Minerals,2003,30(1):44-53. doi: 10.1007/s00269-002-0286-y [32] NEUMANN A,HOFSTETTER T B,LÜSSI M,et al. Assessing the redox reactivity of structural iron in smectites using nitroaromatic compounds as kinetic probes[J]. Environmental Science & Technology,2008,42(22):8381-8387. [33] NEUMANN A,PETIT S,HOFSTETTER T B. Evaluation of redox-active iron sites in smectites using middle and near infrared spectroscopy[J]. Geochimica et Cosmochimica Acta,2011,75(9):2336-2355. [34] MANCEAU A,LANSON B,DRITS V,et al. Oxidation-reduction mechanism of iron in dioctahedral smectites:I. Crystal chemistry of oxidized reference nontronites[J]. American Mineralogist,2000,85(1):133-152. doi: 10.2138/am-2000-0114 [35] FIALIPS C I,HUO D,YAN L,et al. Effect of Fe oxidation state on the IR spectra of Garfield nontronite[J]. American Mineralogist,2002,87(5/6):630-641. [36] MADEJOVá J. FTIR techniques in clay mineral studies[J]. Vibrational Spectroscopy,2003,31(1):1-10. doi: 10.1016/S0924-2031(02)00065-6 [37] YAN L,STUCKI J W. Structural perturbations in the solid-water interface of redox transformed nontronite[J]. Journal of Colloid and Interface Science,2000,225(2):429-439. -
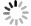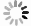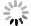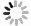



 下载:
下载: